Note: Descriptions are shown in the official language in which they were submitted.
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
IMPROVED BATTERY WITH SPINEL CATHODE
RELATED APPLICATIONS
[0001] This application claims priority to pending U.S. Provisional Appl.
No.
63/090,980 filed October 13, 2020 which is incorporated herein by reference.
BACKGROUND
[0002] The present application is related to an improved method of forming
fine and
ultrafine powders and nanopowders of lithium ion cathodes for batteries and
improved
batteries formed there with. More specifically, the present invention is
related to, but not
limited to, lithium ion battery cathodes and a synergistic electrolyte
formulation which
provides a battery which can withstand many discharge/recharge cycles and
therefore
provides a long battery life without degradation.
[0003] There is an ever-present demand for improvements in batteries. There
are
two primary applications for batteries with one being stationary applications
and the
other being mobile applications. With both stationary and mobile applications
there is a
desire for increased storage capacity, longer battery life, the ability to
reach full charge
quicker and lower cost. Lithium ion batteries, comprising a lithium metal
oxide cathode,
are highly advantageous as a suitable battery for most applications and they
have found
favor across the spectrum of applications. Still, there is a desire for an
improvement in,
particularly, the storage capability, recharge time, cost and storage
stability of lithium ion
batteries. The present invention is focused, primarily on lithium ion
batteries in a spinel
crystalline form or rock-salt crystalline form, on improvements in the
manufacturing
process thereof and a synergistic electrolyte.
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[0004] The preparation of lithium ion batteries comprising lithium and
transition metal
based cathodes in a rock-salt crystalline form are described in U.S. Pat. Nos.
9,136,534; 9,159,999 and 9,478,807 and U.S. Published Pat. Appl. Nos.
2014/0271413;
2014/0272568 and 2014/0272580 each of which are incorporated herein by
reference.
Cathode materials having a rock-salt crystalline form have general formula:
LiNiaMnbXc02
wherein X is preferably Co or Al and a+b+c = 1. When X is cobalt the cathode
materials
are referred to as NMC's, for convenience, and when X is aluminum the cathode
materials are referred to as NCA's, for convenience. In the preparation of the
rock-salt
crystalline form the transition metals can be precipitated as carbonates by
the addition
of a stoichiometric equivalent of lithium carbonate to form cathode material
precursors.
The cathode material precursors are then sintered to form the cathode
material.
[0005] Cathode materials having the spinel crystalline structure have
general
formula:
LiNixMnyCoz04
wherein x+y+z = 2. In the spinels the lithium stoichiometry is half that of
transition metal
stoichiometry. Therefore, the carbonate available from lithium carbonate is
insufficient
to precipitate the transition metals when synthesizing cathode material
precursors. The
addition of excess carbonate can only be achieved through the introduction of
undesirable counterions, such as sodium when sodium carbonate is used, or
complicates pH control and may lead to insufficient precipitation, such as
when
ammonium carbonate is added. A twice stoichiometric excess of lithium
carbonate
could be used in principle, and removed through decantation of the aqueous
2
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
supernatant, however this is undesirable due to the sensitivity of cell
performance with
variation in lithium stoichiometry.
[0006] Spinel cathode materials, such as LiNi0.5Mn1.504, often suffer from
surface
degradation caused by liquid-based electrolyte attack. The result of the
electrolyte
attack is disproportionation of Mn3+. In a cell Mn3+ can disproportionate to a
soluble
Mn2+ species which can contaminate the graphite anode and lead to rapid cell
failure.
This effect is enhanced at high temperature and cell failure can be observed
in less than
100 cycles at C-rate (1 hr discharge). Spinel cathodes, such as
LiNi0.5Mn1.504, are also
good candidates for use with solid-state electrolytes; however, due to the
difference in
Li + diffusion rates between the cathode and electrolyte, a space-charge is
formed at the
interface. The space-charge increases Li-transport resistance within the
electrolyte/electrode interface which is undesirable.
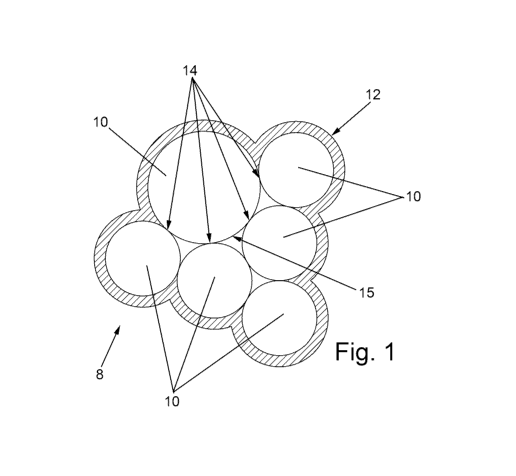
[0007] Without being limited to theory, it is hypothesized that during the
formation of
high nickel NMC's the particles agglomerate. Since this agglomeration occurs
prior to
the formation of the lithium niobate coating the agglomerate is coated as
illustrated
schematically in Fig. 1. In Fig. 1, an agglomerate, 8, of particles, 10, has a
coating, 12,
formed on the surface of the agglomerate. In the interior regions of the
agglomerate
particles have uncoated regions at the interstitial interfaces, 14, between
particles and
at interstitial surfaces, 15, of the particles which are not coated with
lithium niobate. If
the agglomerate is unperturbed the interior uncoated regions are of no
consequence.
Unfortunately, during the process of forming a cathode the particles may at
least
partially de-agglomerate leading to particles with uncoated surface, 11, as
illustrated in
Fig. 2 wherein the uncoated surface may originate from uncoated interstitial
interfaces
3
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
or interstitial surfaces. Yet another perturbation is believed to be the
charging cycle
which is hypothesized to also cause some de-agglomeration or, at least,
sufficient
separation at the particle boundaries to effectively expose uncoated regions
of the
particles. The uncoated region is believed to be a source of degradation of
the high
nickel NMCs, particularly, when utilized with a liquid-based electrolyte.
[0008] There has been a desire for an improved method of manufacturing
lithium ion
cathodes and particularly lithium/manganese/nickel based cathodes in a spinel
and rock
salt crystalline structures. There is a particular desire to provide
lithium/manganese/nickel based cathodes in a spinel comprising a surface
coating
which inhibits degradation, particularly, the degradation which commonly
occurs with
liquid based electrolytes. The present invention provides such a method.
SUMMARY OF THE INVENTION
[0009] It is an object of this invention to provide an improved method of
preparing a
battery comprising a lithium ion cathode and a synergistic electrolyte.
[0010] Another particular feature is the incorporation of a stabilizing
coating on the
surface of the cathode material wherein the coating inhibits degradation,
particularly, the
degradation which occurs by liquid-based electrolyte attack.
[0011] Another feature of the invention is the synergistic combination of,
a preferably
spinel, based cathode and an electrolyte for use with the spinel based cathode
wherein
the cathode and electrolyte are synergistic providing a stability which is
unexpected in
the art.
[0012] An embodiment of the invention is provided in a method of forming a
battery
comprising:
forming a coated lithium ion cathode material comprising:
4
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
in one pot;
forming a first solution comprising a digestible feedstock of a first metal
suitable for
formation of a cathode oxide precursor and a multi-carboxylic acid;
digesting the digestible feedstock to form a first metal salt in solution
wherein the first
metal salt precipitates as a salt of deprotonated multi-carboxylic acid
thereby forming an
oxide precursor wherein the first metal salt comprises lithium and at least
one of Mn, N
Co, Al or Fe;
adding a coating metal precursor salt after digestion; and
heating said oxide precursor to form lithium ion cathode material with an
oxide of
coating metal precursor salt as a coating on lithium ion cathode material; and
providing an anode and an electrolyte wherein said electrolyte has no more
than 1 wt%
of additional salts and additives; and
forming the anode and cathode into a battery with the anode and cathode
separated by
the electrolyte
[0013] Yet another embodiment is provided in a method of forming a battery
comprising:
forming a coated lithium ion cathode material comprising:
in one pot;
reacting lithium carbonate, manganese carbonate and nickel carbonate with
oxalic acid,
liberating CO2(g) and H20(I) to forming a precipitate comprising lithium
oxalate,
manganese oxalate and nickel oxalate to form an oxide precursor;
adding a coating metal precursor salt to the oxide precursor; and
heating the oxide precursor to form the coated lithium ion cathode material;
and
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
providing an anode and an electrolyte wherein said electrolyte has no more
than 1 wt%
of additional salts and additives;
combining the anode and cathode into a battery with the anode and cathode
separated
by the electrolyte.
[0014] Yet another embodiment is provided in an improved lithium ion
battery
comprising:
a cathode comprising particles comprising an oxide defined by the formula:
LiNiaMnbXcGd02
wherein G is an optional dopant;
Xis Co or Al;
a > 0.5;
b+c+d <0.5;
and d 0.1; and
each particle comprises a coating covering a surface of the particle wherein
the coating
comprises a salt of an oxide of a metal selected from the group consisting of
vanadium,
tantalum and niobium; and
an agglomerate comprising the particles wherein the agglomerate comprises
interstitial
interfaces wherein the interstitial interfaces comprise adjacent coatings on
adjacent the
particles;
an anode; and
an electrolyte wherein said electrolyte has no more than 1 wt% of additional
salts and
additives.
BRIEF DESCRIPTION OF THE FIGURES
[0015] Fig. 1 is a schematic representation.
6
CA 03196927 2023-03-27
WO 2022/077105
PCT/CA2021/051436
[0016] Fig. 2 is a schematic representation of the prior art.
[0017] Fig. 3 is a schematic representation.
[0018] Fig. 4 is a schematic representation of an isolated particle
comprising a
coating.
[0019] Fig. 5 provides SEM micrographs of oxalate spray dried precursors
and
LiNia5Mn1.504 material calcined at 900 C for 15 hours when using transition
metal
acetate (top) and carbonate (bottom) feedstocks.
[0020] Fig. 6 provides X-ray Diffraction (XRD) patterns of manganese
oxalate
hydrates precipitated from the reaction of manganese carbonate and oxalic acid
in
water in different conditions.
[0021] Fig. 7 demonstrates improvements in the specific capacity as a
function of
voltage for the spinel material formed by the improved process.
[0022] Fig. 8 is an XRD pattern.
[0023] Fig. 9 is an SEM micrograph.
[0024] Fig. 10 is an XRD pattern.
[0025] Fig. 11 is SEM micrograph.
[0026] Fig. 12 is a graphical representation.
[0027] Fig. 13 is a graphical representation.
[0028] Fig. 14 is a graphical representation.
[0029] Fig. 15 is a graphical representation.
[0030] Fig. 16 is a graphical representation.
[0031] Fig. 17 is an XRD pattern.
[0032] Fig. 18 is a graphical representation.
7
CA 03196927 2023-03-27
WO 2022/077105
PCT/CA2021/051436
[0033] Fig. 19 is a graphical representation.
[0034] Fig. 20 is a graphical representation.
[0035] Fig. 21 is an XRD pattern.
[0036] Fig. 22 is a graphical representation.
[0037] Fig. 23 is a graphical representation.
[0038] Fig. 24 is a graphical representation.
[0039] Fig. 25 is a graphical representation.
[0040] Fig. 26 is a graphical representation.
[0041] Fig. 27 is a graphical representation.
[0042] Fig. 28 is a graphical representation.
[0043] Fig. 29 is an XRD pattern.
[0044] Fig. 30 is an XRD pattern.
[0045] Fig. 31 is an XRD pattern.
[0046] Fig. 32 is an XRD pattern.
[0047] Fig. 33 is a SEM micrograph.
[0048] Fig. 34 is an XRD pattern.
[0049] Fig. 35 is an XRD pattern.
[0050] Fig. 36 is SEM micrograph.
[0051] Fig. 37 is SEM micrograph of an embodiment of the invention.
[0052] Fig. 38 is a graphical representation.
[0053] Fig. 39 is an XRD pattern.
[0054] Fig. 40 is an XRD pattern.
[0055] Fig. 41 is SEM micrograph.
8
CA 03196927 2023-03-27
WO 2022/077105
PCT/CA2021/051436
[0056] Fig. 42 is SEM micrograph.
[0057] Fig. 43 is a graphical representation.
[0058] Fig. 44 is a graphical representation.
[0059] Fig. 45 is an XRD pattern.
[0060] Fig. 46 is a graphical representation.
[0061] Fig. 47 is a graphical representation.
[0062] Fig. 48 is an XRD pattern.
[0063] Fig. 49 is SEM micrograph.
[0064] Fig. 50 is a graphical representation.
[0065] Fig. 51 is an XRD pattern.
[0066] Fig. 52 is SEM micrograph.
[0067] Fig. 53 is an XRD pattern.
[0068] Fig. 54 is SEM micrograph.
[0069] Fig. 55 is a graphical representation.
[0070] Fig. 56 is a graphical representation.
[0071] Fig. 57 is a graphical representation.
[0072] Fig. 58 is an XRD pattern.
[0073] Fig. 59 is an SEM micrograph.
[0074] Fig. 60 is a graphical representation.
[0075] Fig. 61 is a graphical representation.
[0076] Fig. 62 is a graphical representation.
[0077] Fig. 63 is a graphical representation.
DESCRIPTION
9
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[0078] The instant invention is specific to an improved method for
preparing a lithium
ion battery, and particularly the cathode of a lithium ion battery. More
particularly, the
present invention is specific to an improved process for forming cathodes for
use in a
lithium ion battery with a synergistic electrolyte wherein the cathode is in a
spinel
crystalline form or a rock-salt form with preferred rock salt forms being NMC
and NCA
materials. Even more specifically, the present invention is specific to the
formation of a
cathode with a lithium ion battery with a synergistic electrolyte wherein the
process
forms the cathode comprising a coating which inhibits the formation of space-
charge
regions at the surface and, more preferably, the coating can be formed in
concert with
the formation of the cathode material in a common pot.
[0079] A particular advantage of the invention is the ability to utilize
electrolytes
which are void of typical salts and additives typically utilized in
electrolytes. Contrary to
conventional expectations the typical salts and additives used in electrolytes
as
stabilizers, and the like, are detrimental to cathodes, particularly spinels,
of the instant
invention. Particularly preferred electrolytes comprise LiPF6 in a solvent
wherein the
solvent is preferably an alkyl carbonate selected from ethylene carbonate
(EC); dimethyl
carbonate (DMC); diethyl carbonate (DEC); ethyl methyl carbonate (EMC); 1,2-
dimethoxyethane; 1,3-dioxolane; acetonitrile; ethyl acetate; fluoroethylene
carbonate;
propylene carbonate and tetrahydrofuran with combinations of ethylene
carbonate (EC);
dimethyl carbonate (DMC); diethyl carbonate (DEC) and ethyl methyl carbonate
(EMC)
being most preferred.
[0080] The LiPF6 is preferably at least 0.1 M to no more than 10 M. Below
about 0.1
M the conductivity is insufficient to function adequately. Above about 10 M
solubility
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
becomes a concern and salt can precipitate from the solution. Most preferably
the
electrolyte comprises about 0.8 to no more than 1.2 M LiPF6 with about 1.0 M
being
optimum.
[0081] The solvent for the electrolyte preferably comprises EC with at
least one of
DMC, DEC or EMC as a co-solvent. It is preferable that the solvent comprise at
least
20 wt% EC to no more than 80 wt% EC with the balance being DMC, DEC, EMC or
combinations thereof. More preferably the solvent comprises at least 30 wt% EC
to no
more than 70 wt% EC with the balance being DMC, DEC, EMC or combinations
thereof.
Approximately equal wt% of EC and DMC, DEC, EMC or combinations thereof is
particularly suitable.
[0082] It is preferable that the electrolyte contain no more than 1 wt% of
additional
salts and additives. More preferably the electrolytes comprise no more than
0.5 wt% of
additional salts and additives and preferably no detectable amount of
additional salts
and additives. Salts and additives preferably avoided include lithium bis
(trifluoromethanesulfonyl) imide; lithium hexafluorophosphate; lithium
perchlorate;
lithium tetrafluoroborate; lithium trifluoromethane sulfonate; tetraethyl-
ammonium
tetrafluoroborate; biphenyl; propane sultone; vinylene carbonate; methyl
ethylene
carbonate; lithium bis(oxalate) borate; lithium difluoro oxalate borate;
lithium
bis(fluorosulfonyl) imide, fluoroethylene carbonate; difluoroethylene
carbonate; succinic
anhydride and ethylene sulfate.
[0083] The particles of the cathode material are coated with a metal oxide
of
niobium, vanadium or tantalum with lithium niobate (LiNb03) being most
preferred. The
coating provides a passivation layer which prevents degradation particularly
when using
11
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
a liquid-based electrolyte such as ethylene carbonate (EC):diethylene
carbonate (DEC)
1:1 and decreases the space-charge resistance when using a solid-state
electrolyte.
[0084] An embodiment of the invention will be described with reference to
Fig. 3
which forms an integral non-limiting component of the invention. In Fig. 3, an
agglomerate, 16, is illustrated schematically in cross-sectional view. The
agglomerate
comprises particles, 10, wherein the entire surface of the particle is coated
with a
protective coating, 12. A result of the entire surface being coated is the
advantage that
the interstitial interfaces, 14, are interfaces comprising coating and the
interstitial
surfaces, 15, are surfaces comprising coating. If any perturbation disturbs
the
agglomerate each particle has a completely coated surface as illustrated
schematically
in Fig. 4 wherein a completely dissociated particle is shown to have a
complete surface
coating. Complete dissociation of a particle is illustrated in Fig. 4 for the
purposes of
discussion with the understanding that most of the perturbations expose
surfaces of the
particles, or in the instant invention the coating on the particles, without
necessarily
complete dissociation of the particles. For the purposes of illustration and
discussion
the coatings of adjacent particles are illustrated as distinct and
distinguishable. In an
actual sample the coatings may form a homogenous layer between adjacent
particles
without the ability to necessarily distinguish a defined barrier between the
coatings of
adjacent particles. In other words, the coating may be distinguishable by
visual and
spectroscopic techniques as being distinct coatings or the coatings may appear
as a
continuum of coating material.
[0085] For the purposes of this disclosure interstitial interfaces of an
agglomerate are
defined as points of contact of adjacent particles, points of contact of the
coating of
12
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
adjacent particles or points of contact of a particle with the coating of an
adjacent
particle. For the purposes of this disclosure interstitial surfaces of an
agglomerate are
defined as a surface of a particle, or the surface of the coating of a
particle, which is not
in contact with an adjacent particle or the coating of an adjacent particle.
[0086] The coating has a preferred thickness of 5 to 10 nanometers over the
entirety
of the particles.
[0087] In a preferred embodiment, the lithium metal compound of the instant
invention comprises lithium metal compound in a spinel crystal structure
defined by the
Formula I:
LiNixMnyCozEw04
Formula I
wherein E is an optional dopant; and
x+y+z+w = 2 and w 0.2; or
a rock-salt crystal structure defined by Formula II;
LiNiaMnbXcGd02
Formula II
wherein G is an optional dopant;
X is Co or Al; and
wherein a+b+c+d = 1 and d 0.1.
[0088] In a preferred embodiment in the spinel crystal structure of Formula
I has 0.5
x 0.6; 1.4 y 1.5 and z 0.9. More preferably 0.5 x 0.55, 1.45 y 1.5 and z
0.05. In a preferred embodiment neither x nor y is zero. In Formula I it is
preferable
that the Mn/Ni ratio is no more than 3, preferably at least 2.33 to less than
3 and most
preferably at least 2.6 to less than 3.
13
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[0089] In a preferred embodiment in the rock-salt crystal structure of
Formula II is a
high nickel NMC wherein 0.5 a 0.9 and more preferably 0.58 a 0.62 as
represented by NMC 622 or 0.78 a 0.82 as represented by NMC 811. In a
preferred
embodiment a=b=c as represented by NMC 111.
[0090] In the formulas throughout the specification, the lithium is defined
stoichiometrically to balance charge with the understanding that the lithium
is mobile
between the anode and cathode. Therefore, at any given time the cathode may be
relatively lithium rich or relatively lithium depleted. In a lithium depleted
cathode the
lithium will be below stoichiometric balance and upon charging the lithium may
be above
stoichiometric balance. Likewise, in formulations listed throughout the
specification the
metals are represented in charge balance with the understanding that the metal
may be
slightly rich or slightly depleted, as determined by elemental analysis, due
to the inability
to formulate a perfectly balanced stoichiometry in practice. Throughout the
specification
specifically recited formulations such as those represented by Formula I and
Formula II,
or specific embodiments thereof, are intended to represent the molar ratio of
the metals
within 10%. For LiNi0.5Mno.3Coo.202, for example, each metal is stated within
10% of
stoichiometry and therefore Nio.5 represents Ni0.45t0 Nio.55.
[0091] Dopants can be added to enhance the properties of the oxide such as
electronic conductivity and stability. The dopant is preferably a
substitutional dopant
added in concert with the primary nickel, manganese and optional cobalt or
aluminum.
The dopant preferably represents no more than 10 mole% and preferably no more
than
mole % of the oxide. Preferred dopants include Al, Gd, Ti, Zr, Mg, Ca, Sr, Ba,
Mg, Cr,
Cu, Fe, Zn, V, Bi, Nb and B with Al and Gd being particularly preferred.
14
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[0092] The cathode is formed from an oxide precursor comprising salts of
Li, Ni, Mn,
Co, Al or Fe as will be more fully described herein. The oxide precursor is
calcined to
form the cathode material as a lithium metal oxide.
[0093] The spinel cathode material, and particularly LiNia5Mn1.504, is
preferably
coated with a metal oxide, most preferably lithium niobate (LiNb03), in the
same pot as
the spinel formation which is referred to herein as a one-pot synthesis. This
process
produces a passivation later of LiNb03 which prevents dissolution of Mn2+ when
using a
liquid-based electrolyte such as ethylene carbonate (EC):diethylene carbonate
(DEC)
1:1 and decreasing the space-charge resistance when using a solid-state
electrolyte.
Through the one-pot synthesis it is hypothesized that niobium prefers to
surface
segregate rather than being doped within the spinel structure due to the
niobium being
in the 5+ oxidation state and having a higher molecular weight than the other
transition
metals.
[0094] The oxide precursors are formed by the reaction of salts in the
presence of
counterions which form relatively insoluble salts. The relatively insoluble
salts are
believed to form suspended crystals which are believed to Ostwald ripen
ultimately
precipitating as an ordered lattice. For the purposes of the present invention
salts of
preferably manganese and nickel, and optionally cobalt or aluminum, combined
in a
solution comprising counterions which precipitate the manganese, nickel and
cobalt or
aluminum at a rate sufficient to allow crystalline growth. Soluble counterions
of
manganese, nickel, cobalt or aluminum are those having a solubility of at
least 0.1 g of
salt per 100 gram of solvent at 20 C including acetate, nitrate or hydrogen
carbonate.
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
The metals are precipitated as insoluble salts having a solubility of less
than 0.05 g of
salt per 100 gram of solvent at 20 C including carbonates and oxalates.
[0095] The overall reaction comprises two secondary reactions, in sequence,
with
the first reaction being the digestion of carbonate feedstock in the presence
of an
excess of multi-carboxylic acid as represented by Reaction A:
XC03(s) + 2H+(aq) X2+ + CO2(g) + H20(1) A
wherein X represents a metal suitable for use in a cathode material preferably
chosen
from Li2, Mn, Ni, Co or Al. In Reaction A the acid is liberated by the multi-
carboxylic
acid which is not otherwise represented in Reaction A for simplicity. The
result of
Reaction A is a metal salt in solution wherein the salt is chelated by the
deprotonated
multi-carboxylic acid as represented by Reaction B:
X2+ + -00CRi C00- ¨> X(00CRi COO)
wherein R1 represents an alkyl chain comprising the multi-carboxylate. The
salts
represented by X(00CRi COO) precipitate in an ordered lattice as discussed
elsewhere
herein.
[0096] The metal carbonates of Reaction A can be substituted with metal
acetates
such as Li(02CCH3), Ni(02CCH3)2 or Mn(02CCH3)2which can be added as aqueous
solutions or as solid materials.
[0097] The pH may be adjusted with ammonium hydroxide, if desired, due to
the
simplicity and improved ability to accurately control the pH. In the prior art
processes
the use of ammonium hydroxide caused difficulty due to the propensity for NH3
to
complex with nickel in aqueous solution as represented by the reaction:
[Ni(H20)6]2+ + x NH3 [Ni(NH3)x(H20)6-d2+ + X H20
16
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
The result is incomplete precipitation of nickel which complicates
determination and
control of stoichiometry of the final oxide precursor. Multi-carboxylic acids,
and
particularly oxalic acid, effectively coordinates nickel preferentially over
NH4 + thereby
increasing the rate of precipitation and incorporation of nickel into the
ordered oxide
precursor. Preferential precipitation by multi-carboxylic acids drives the
reaction
towards nickel precipitation and avoids the use of ammonium hydroxide.
[0098] A particularly preferred embodiment is represented by the formation
of
LiNia5Mn1.504 from the oxide precursor represented by the, preferably aqueous,
reaction:
0.5 Li2CO3 + 0.5 NiCO3 + 1.5 MnCO3 + 2.5 H2C204
0.5 Li2C204 + 0.5 NiC204 + 1.5 MnC204 + 2.5 CO2 + 2.5 H20
wherein the, NiC204, and MnC204 precipitate in an ordered lattice as an oxide
precursor
with Li2C204 precipitated thereon upon removal of water. The oxide precursor
having
the gross composition (Li2C204)0.5(NiC204)0.5(MnC204)1.5 is calcined resulting
in the
reaction:
(Li2C204)0.5(NiC204)0.5(MnC204)1.5 202 LiNia5Mn1.504+ 5CO2
[0099] The carbonate digestion process in the presence of multi-carboxylic
acids
includes combining the metal carbonate and oxalic acid into a reactor,
preferably in the
presence of water, followed by stirring. The slurry is then dried, preferably
by spray
drying, followed by calcining. The calcination temperature can vary from 400
to 1000 C
to form materials with different structural properties, for example, different
degrees of
Mn/Ni cation ordering in spinel LiNi0.5Mn1.504.
17
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00100] A particular feature of the carbonate digestion process is the fact
that there is
no need to grind or blend the precursor powders, filter the slurry, or decant
the
supernatant even though these steps can be done if desired.
[00101] The carbonate digestion process or digestion(hydrolysis)-precipitation
reaction, using oxalate as an example, can be described by the following
equation
which occurs preferably in the presence of water:
H2C204(aq) XCO3 (s) 4 CO2(g) H20(1) XC204(s,aq) (X = transition metals,
Li2)
[00102] Without being limited to theory, it is hypothesized that the oxalic
acid
hydrolyses the carbonates to form CO2(g), H20(I), and metal ions. Transition
metal ions
are then precipitated as metal oxalates. Lithium oxalate might be precipitated
or remain
soluble in water, depending on the water content. The soluble lithium oxalate
is
expected to be coated on transition metal oxalate particles during spray-
drying. There is
no need to achieve complete dissolution of metal carbonates or oxalic acid as
the water
is simply a medium to digest the metal carbonates and precipitate out the
metal
oxalates in a controlled fashion thereby allowing for nucleation and crystal
growth. The
rate of the digestion(hydrolysis)-precipitation reaction depends on
temperature, water
content, pH, gas introduction, the crystal structure and morphology of the
feedstocks.
[00103] The reaction can be completed in the temperature range of 10¨ 100 C
with
water reflux temperature being preferred in one embodiment due to the
increased
digestion reaction rate.
[00104] For each lg of oxalic acid the water content can vary from about 1 to
about
400 ml with a preference for a decreased water content due to the increased
reaction
rate and less water must be removed subsequently.
18
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00105] The pH of the solution can vary from 0 to 12. A particular advantage
of the
carbonate digestion process is that the reaction can be done without
additional pH
control thereby simplifying the process and eliminating the need for
additional process
control or additions.
[00106] Whereas the reaction can be done under untreated atmospheric air other
gases such as CO2, N2, Ar, other inert gases or 02 can be used in some
embodiments.
In some embodiments N2 and CO2 bubbling into the solution are preferred as
they may
slightly increase the crystallinity of the precipitated metal oxalates.
[00107] The crystallinity and morphology of the precursors, such as amorphous
vs.
crystalline carbonate feedstocks can influence the rate of digestion due to
the
differences in solubility and particle size and range of particle size.
[00108] The carbonate digestion process proceeds via a cascading equilibrium
from
solid carbonate feedstocks to solid oxalate precursor materials. The process
can be
defined by several distinct processes, per the following reactions, for the
purposes of
discussion without limit thereto:
(1) H2C204(s) 4 H2C204(ac) (dissolution of oxalic acid)
(2) H2C204(ac) E H(ac) + HC204-(aq) (oxalic acid dissociation step one, pKa
= 1.25)
(3) HC204-(ac) E H(ac) + C2042(ac) (oxalic acid dissociation step two, pKa
= 4.19)
(4) XC03(s, aq) 2H+(aq) 4 X2+ + H20(I) + CO2(g) (carbonate hydrolysis)
(5) X2(ac) + C2042(ac) 4 XC204(s) (precipitation of metal oxalates)
[00109] If this reaction were to be used to create the high voltage
LiNia5Mn1.504
material, the following reaction would be take place which would be
preferably, but not
necessarily, in the presence of H20:
19
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
(6) 0.25Li2CO3(s) + 0.25NiCO3(s) + 0.75MnCO3(s) + 1 .25H2C204(ac) 4
0.25Li2C204(ac) + N io.25Mno.75C204(s) + 1 .25CO2(g) + 1 .25H20(I)
[00110] For the purposes of discussion and explanation the reactions are
written
stepwise with the understanding that under operational reaction conditions the
reactions
may be occurring simultaneously. By varying different reaction parameters such
as
water content/ionic strength, excess oxalic acid content, batch size,
temperature,
atmosphere, refluxing the reaction mixture, pH control, etc. the rates of each
step can
be controlled and other desirable parameters such as solids content can be
optimized.
[00111] The carbonate digestion process can be described as proceeding in a
cascading equilibrium as the evolution of CO2(g)from solution, as in Reaction
4 above,
and precipitation of highly insoluble metal oxalates, as in Reaction 5 above.
Both CO2
evolution and precipitation drive the reaction to completion.
[00112] Rates of carbonate hydrolysis are correlated to Ksp of the metal
carbonate
with the following provided for convenience:
Lithium carbonate, Li2CO3, 8.15x10-4 Very fast (seconds to minutes);
Nickel(11) carbonate, NiCO3, 1.42x10-7 Fast (minutes);
Manganese(II) carbonate, MnCO3, 2.24x10-11 Slower (hours to days); and
Aluminum hydroxide (Al(OH)3, 3x10-34 Very slow
[00113] The homogeneity of co-precipitation could depend on rates of carbonate
hydrolysis. For example, if Nickel(11) carbonate is fully hydrolyzed before
Manganese(II)
carbonate, it may subsequently precipitate as NiC204 and MnC204 separately.
[00114] Temperature can be controlled as it influences the rates of
dissolution of
oxalic acid, carbonate hydrolysis, and precipitation of metal oxalates.
Specifically, it
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
would be useful to perform the reaction at water reflux temperature. CO2(g) is
produced
in this reaction, and raising the temperature will increase the rate of
removal of CO2(g),
and therefore due to lower aqueous CO2(g) solubility at high temperatures
increasing the
temperature may increase the rate of carbonate hydrolysis.
[00115] Gas bubbling may also be an effective method of controlling the rates
of
reaction by altering the rate of CO2 evolution. Bubbling of N2(g), 02(g),
CO2(g), and/or
atmospheric air may be beneficial as the gases may function to displace
dissolved
CO2(g) or improve mixing of reactants.
[00116] The carbonates may digest faster if they are first in the form of the
metastable
bicarbonate. For example, the following reaction occurs for Li2CO3:
Li2CO3(s) + CO2(g) H20(1) 2 LiHCO3(ac)
[00117] The metastable lithium bicarbonate is far more soluble than Li2CO3 and
the
subsequent hydrolysis can proceed stoichiometrically with a single proton as
shown
below:
LiHCO3(ac) + H+(aq) 4 H20(I) + CO2(g) Li+(aq)1
as opposed to proceeding as Reaction 4 above.
[00118] Divalent metal oxalates such as NiC204, MnC204, CoC204, ZnC204, etc.
are
highly insoluble, however monovalent metal oxalates such as Li2C204 are
somewhat
soluble with a solubility of 8g/100m L at 25 C in water. If it is necessary to
have the
lithium oxalate in solution and homogeneously dispersed throughout a mixed
metal
oxalate precipitate, then keeping the water volume above the solubility limit
of lithium
oxalate may be advantageous.
21
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00119] The rates of carbonate hydrolysis, metal oxalate precipitation, and
the crystal
structure and particle size of the metal oxalate precipitate is influenced by
pH and water
content or ionic strength. In some embodiments it may be beneficial to work at
higher
ionic strength, or lower water content as this increases the proton activity
of oxalic acid,
and rates of precipitation of metal oxalates. Water content can be normalized
to
carbonate feedstock content with a preferred ratio of moles of carbonates to
volume of
water in L being in the range of about 0.05 to about 20. A water content of
about 1.64 L
per 1.25 moles of carbonates provides a ratio of moles of carbonates to volume
of water
in L of 1.79 which is suitable for demonstration of the invention.
[00120] A stoichiometric amount of oxalate to carbonate is sufficient to
achieve
complete precipitation. However, adding excess oxalic acid can increase the
reaction
rate as the second proton on oxalic acid is much less acidic and is involved
in the
hydrolysis. About 5% excess oxalic acid by mole to carbonates is sufficient to
ensure
completion of carbonate hydrolysis. ICP analyses have shown that 10% excess
oxalic
acid leaves a similar number of Mn/Ni ions in solution as 0% stoichiometric
excess by
the completion of the reaction. A small stoichiometric excess of oxalic acid
should be
effective in achieving complete precipitation however a low stoichiometric
excess may
impact the rate of carbonate hydrolysis.
[00121] A particular advantage of the carbonate digestion process is the
ability to do
the entire reaction in a single reactor until completion. As the lithium
source is ideally in
solution prior to the spray drying and calcination steps, it may be useful
and/or possible
to precipitate the transition metals separately and to add the lithium source
after co-
precipitation as a solution of an aqueous lithium salt such as oxalate.
22
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00122] A coating metal precursor salt, wherein the metal will not incorporate
into the
lattice, can be added after digestion to eventually form the metal oxide
coating. A
particularly preferred metal is niobium and a particularly preferred niobium
precursor, as
the coating metal precursor salt, is a dicarboxylic acid salt with oxalate
being most
preferred. The preferred niobium oxalate can be formed in-situ from niobium
carbonate
or niobium oxalate can be prepared separately and added to the cathode metal
precursors. It is preferable that the coating comprise predominantly the
coating
material as a lithium salt, preferably lithium niobate, wherein at least 95
mole percent of
the coating is the lithium salt of the coating metal oxide or less than 5 mole
percent of
the metal ion in the coating is a lithium salt of an active cathode material
as defined in
Formula I or Formula II. In a particularly preferred embodiment the metal in
the coating
is at least 95 mole percent lithium niobate.
[00123] The invention is suitable for use with transition metal acetates and
mixed
carbonate feedstocks thereby allowing the solubility of the metal complexes to
be more
closely matched. Mixed carbonate feedstock such as Ni0.25Mn0.75CO3 + Li2CO3 to
produce a LiNi0.5Mn1.504 material are contemplated. Feedstock impurities may
be
critical to the performance of final materials. In particular, samples of
MnCO3 may have
small quantities of unknown impurities which are not hydrolyzed during
refluxing.
[00124] Multi-carboxylic acids comprise at least two carboxyl groups. A
particularly
preferred multi-carboxylic acid is oxalic acid due, in part, to the
minimization of carbon
which must be removed during calcining. Other low molecular weight di-
carboxylic
acids can be used such as malonic acid, succinic acid, glutaric acid and
adipic acid.
Higher molecular weight di-carboxylic acids can be use, particularly with an
even
23
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
number of carbons which have a higher solubility, however the necessity of
removing
additional carbons and decreased solubility renders them less desirable. Other
acids
such as citric, lactic, oxaloacetic, fumaric, maleic and other polycarboxylic
acids can be
utilized with the proviso they have sufficient solubility to achieve at least
a small
stoichiometric excess and have sufficient chelating properties. It is
preferable that acids
with hydroxyl groups not be used due to their increased hygroscopic
characteristics.
[00125] To accomplish the reaction to form the oxide precursor solutions of
the
starting salts are prepared. It is preferable to prepare added solutions,
preferably
comprising the nickel, manganese and cobalt or aluminum solutions either
collectively,
separately, or in some combination, and a bulk solution preferably comprising
the
lithium. The added solution is then added, as described elsewhere herein, to
the bulk
solution. The solutions can be reversed, however, it is preferable that the
transition
metals be added in the intended stoichiometry and it is therefore advantageous
to add
as a single solution comprising all transition metals to a lithium containing
bulk solution.
[00126] Each solution is prepared by dissolving the solid in a selected
solvent,
preferably a polar solvent, such as water, but not limited thereto. The choice
of the
solvent is determined by the solubility of the solid reactant in the solvent
and the
temperature of dissolution. It is preferred to dissolve at ambient temperature
and to
dissolve at a fast rate so that solubilization is not energy intensive. The
dissolution may
be carried out at a slightly higher temperature but preferably below 100 C.
Other
dissolution aids may be addition of an acid or a base.
[00127] During mixing it is preferable to bubble gas into the bulk solution.
For the
purposes of discussion the gas is defined as inert, which has no contribution
to the
24
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
chemical reaction, or the gas is defined as reactive, which either adjust the
pH or
contributes to the chemical reaction. Preferred gases include air, CO2, NH3,
SF6, HF,
HCI, N2, helium, argon, methane, ethane, propane or mixtures thereof. A
particularly
preferred gas includes ambient air unless the reactant solutions are air-
sensitive.
Carbon dioxide is particularly preferred if a reducing atmosphere is required
and it can
also be used as a dissolution agent, as a pH adjusting agent or as a reactant
if
carbonates are formed. Ammonia may also be introduced as a gas for pH
adjustment.
Ammonia can form ammonia complexes with transition metals and may assist in
dissolving such solids. Mixtures of gases may be employed such as 10% 02 in
argon
as an example.
[00128] For the formation of the oxide precursor the pH is preferably at least
about 1
to no more than about 9.6 without limit thereto. Ammonia, or ammonium
hydroxide, is
suitable for increasing pH as is any soluble base with LiOH being particularly
preferred
for adjustment if necessary. Acids, particularly formic acid, are suitable for
decreasing
pH if necessary. In one embodiment lithium can be added, such as by addition
of
lithium acetate to achieve adequate solids content, typically about 20 to 30
wt%, prior to
drying.
[00129] A particular advantage of the instant invention is the ability to form
gradients
of transition metal concentration throughout the body of the oxide wherein
regions, the
center for example, can have one ratio of transition metals and that ratio can
vary in
either continuous fashion or step-wise fashion through the body of the oxide.
Considering NMC for the purposes of discussion and clarification without limit
thereto,
the concentration of Ni, Mn and Co can change radially from the core towards
the
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
surface of a particle. In an exemplary embodiment provided for clarity, the Ni
content
can be in a gradient thereby allowing a relatively low nickel concentration on
or near the
surface of the oxide particle and relatively high nickel concentration in the
core of the
oxide particle. The ratio of Li to transition metals would remain constant,
based on
neutral stoichiometry, throughout the oxide particle. By way of clarifying
example, the
overall compositions of Ni:Mn:Co may be 6:2:2 and 8:1:1 for NMC 622 and NMC
811,
respectively, with the core being relatively rich in one transition metal and
the shell
being relative poor in the same transition metal. Even more specifically, the
core may
be rich in one transition metal, nickel for example, with a radially
decreasing ratio in that
transition metal relative to the others. An NMC 8:1:1 core, for example may
have
exterior thereto an NMC 6:2:2 shell with an NMC 1:1:1 shell on the exterior as
a non-
limiting step-wise example. These reactions can be done in step-wise
additions, or in a
continuous gradient by altering the pump rates of the transition metals. The
ratio of
transition metals in each addition and the number of additions can be altered
to obtain
desired gradient distributions.
[00130] A particular feature of the instant invention is the ability to
incorporate dopants
and other materials either preferentially in the interior of the oxide or
towards the
surface or even at the surface. With prior art techniques dopants, for
example, are
homogenously dispersed within the oxide. Furthermore, any surface treatment,
such as
with aluminum, is on a formed oxide as a surface reactant not necessarily as
an atom
incorporated into the oxide lattice. The present invention allows dopants to
be
dispersed systematically at the core, as would be the case if the dopant were
incorporated into the initial transition metal slurry, in a radial band, as
would be the case
26
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
if the dopant were incorporated into a subsequent transition metal slurry, or
in an outer
shell, as would be the case if the dopant were incorporated into the final
transition metal
slurry.
[00131] For the purposes of the instant invention, each radial portion of the
oxide
particle will be defined based on the percentage of transition metal used to
form the
portion. By way of example, if the initial slurry has a first ratio of
transition metals, and
the initial slurry comprises 10 mol% of the total transition metal used to
form the oxide,
the core will be considered to be 10 (:)/0 of the volume of the oxide and the
composition of
the core will be defined as having the same ratio as the first ratio of
transition metals.
Similarly, each shell surrounding the core will be defined by the percentage
of transition
metal therein. By way of non-limiting example, a precursor to the oxide formed
with
three slurries, each of equal moles of transition metal, wherein the first
slurry had a
Ni:Mn:Co ratio of 8:1:1, the second slurry had a Ni:Mn:Co ratio of 6:2:2 and
the third
slurry had a Ni:Mn:Co ratio of 1:1:1 would be considered to form an oxide
representing
1/3 of the volume of the oxide particle being a core with transition metals in
the ratio of
8:1:1, a first shell on the core representing 1/3 of the volume of the oxide
particle with a
transition metal ratio of 6:2:2 and an outer shell on the first shell
representing 1/3 of the
volume of the oxide particle with a transition metal ratio of 1:1:1 without
regards for the
migration of transition metals which may occur during sintering of the
precursor to the
oxide.
[00132] In a particularly preferred embodiment, a dopant is incorporated into
an outer
shell with a particular dopant being aluminum. More preferably, the outer
shell
comprising the dopant represents less than 10 (:)/0 of the volume of the oxide
particle,
27
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
even more preferably less than 5 A of the volume of the oxide particle and
most
preferably no more than 1 A of the volume of the oxide particle. For the
purposes of
the present invention a dopant is defined as a material precipitated during
the formation
of the precursor to the oxide in concert with at least one transition metal
selected from
Ni, Mn, Co, Al and Fe. More preferably, the precursor to the oxide comprises
Ni and Mn
and optionally either Co or Al. A material added after completion of the
precipitation of
at least one transition metal is defined herein as a surface treatment with
niobium, and
particularly lithium niobate, being preferred.
[00133] Upon completion of the reaction to form the oxide precursor, the
resulting
slurry mixture is dried to remove the solvent and to obtain the dried
precursor powder.
Any type of drying method and equipment can be used including spray dryers,
tray
dryers, freeze dryers and the like, chosen depending on the final product
preferred. The
drying temperatures would be defined and limited by the equipment utilized and
such
drying is preferably at less than 350 C and more preferably 200-325 C. Drying
can be
done using an evaporator such that the slurry mixture is placed in a tray and
the solvent
is released as the temperature is increased. Any evaporator in industrial use
can be
employed. A particularly preferred method of drying is a spray dryer with a
fluidized
nozzle or a rotary atomizer. These nozzles are preferably the smallest size
diameter
suitable for the size of the oxide precursor in the slurry mixture. The drying
medium is
preferably air due to cost considerations.
[00134] The particle sizes of the oxide precursor are of nanosize primary and
secondary particles and up to small micron size secondary particles ranging to
less than
50 micron aggregates which are very easily crushed to smaller size. It should
be known
28
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
that the composition of the final powder influences the morphology as well.
The oxide
precursor has a preferred particle size of about 1-5 pm. The resulting mixture
is
continuously agitated as it is pumped into the spray dryer head if spray
dryers, freeze
dryers or the like are used. For tray dryers, the liquid evaporates from the
surface of the
solution.
[00135] The dried powders are transferred into the calcining system batch-wise
or by
means of a conveyor belt. In large scale production, this transfer may be
continuous or
batch. The calcining system may be a box furnace utilizing ceramic trays or
saggers as
containers, a rotary calciner, a fluidized bed, which may be co-current or
counter-
current, a rotary tube furnace and other similar equipment without limit
thereto.
[00136] The heating rate and cooling rate during calcinations depend on the
type of
final product desired. Generally, a heating rate of about 5 C per minute is
preferred but
the usual industrial heating rates are also applicable.
[00137] The final powder obtained after the calcining step is a fine,
ultrafine or
nanosize powder that may not require additional crushing, grinding or milling
as is
currently done in conventional processing. Particles are relatively soft and
not sintered
as in conventional processing.
[00138] The final calcined oxide powder is preferably characterized for
surface area,
particle size by electron microscopy, porosity, chemical analyses of the
elements and
also the performance tests required by the preferred specialized application.
[00139] The spray dried oxide precursor is preferably very fine and nanosize.
[00140] A modification of the spray dryer collector such that an outlet valve
opens and
closes as the spray powder is transferred to the calciner can be implemented.
29
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
Batchwise, the spray dried powder in the collector can be transferred into
trays or
saggers and moved into a calciner. A rotary calciner or fluidized bed calciner
can be
used to demonstrate the invention. The calcination temperature is determined
by the
composition of the powder and the final phase purity desired. For most oxide
type
powders, the calcination temperatures range from as low as 400 C to slightly
higher
than 1000 C. After calcination, the powders are sieved as these are soft and
not
sintered. The calcined oxide does not require long milling times nor
classifying to obtain
narrow particle size distribution.
[00141] The LiM204 spinel oxide has a preferred crystallite size of 1-5 m. The
LiM02
rock salt oxide has a preferred crystallite size of about 50-250 nm and more
preferably
about 150-200 nm.
[00142] A particular advantage of the present invention is the formation of
metal
chelates of multi-carboxylic acids as opposed to acetates. Acetates function
as a
combustion fuel during subsequent calcining of the oxide precursor and
additional
oxygen is required for adequate combustion. Lower molecular weight multi-
carboxylic
acids, particularly lower molecular weight dicarboxylic acids, and more
particularly
oxalic acid, decompose at lower temperatures without the introduction of
additional
oxygen. The oxalates, for example, decompose at about 300 C, without
additional
oxygen, thereby allowing for more accurate control of the calcining
temperature. This
may allow for reduced firing temperatures thereby facilitating the formation
of disordered
Fdgm spinel crystalline structures with minimal impurity phase occurring as
seen at high
temperature
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00143] This method for forming the oxide precursor is referred to herein as
the
complexometric precursor formulation (CPF) method which is suitable for large
scale
industrial production of high performance fine, ultrafine and nanosize powders
requiring
defined unique chemical and physical properties that are essential to meet
performance
specifications for specialized applications. The CPF method provides an oxide
precursor wherein the metals are precipitated as salts into an ordered
lattice. The oxide
precursor is then calcined to form the oxide. While not limited to theory, it
is
hypothesized that the formation of an ordered lattice, as opposed to an
amorphous
solid, facilitates oxide formation during calcination.
[00144] The CPF method provides for the controlled formation of specialized
microstructures or nanostructures and a final product with particle size,
surface area,
porosity, phase purity, chemical purity and other essential characteristics
tailored to
satisfy performance specifications. Powders produced by the CPF method are
obtained
with a reduced number of processing steps relative to currently used
technology and
can utilize presently available industrial equipment.
[00145] The CPF method is applicable to any inorganic powder and
organometallic
powders with electrophilic or nucleophilic ligands. The CPF method can use low
cost
raw materials as the starting raw materials and if needed, additional
purification or
separation can be done in-situ. Inert or oxidative atmospheric conditions
required for
powder synthesis are easily achieved with the equipment for this method.
Temperatures
for the reactions can be ambient or slightly warm but preferably not more than
100 C.
[00146] The CPF method produces fine, ultrafine and nanosize powders of
precursor
oxides in a simple efficient way by integrating chemical principles of
crystallization,
31
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
solubility, transition complex formation, phase chemistry, acidity and
basicity, aqueous
chemistry, thermodynamics and surface chemistry.
[00147] The time when crystallization begins and, in particular, when the
nucleation
step begins, is the most crucial stage of formation of nanosize powders. A
particular
advantage provided by CPF is the ability to prepare the nanosize particles at
the onset
of this nucleation step. The solute molecules from the starting reactants are
dispersed in
a given solvent and are in solution. At this instance, clusters are believed
to begin
forming on the nanometer scale under the right conditions of temperature,
supersaturation, and other conditions. The clusters constitute the nuclei
wherein the
atoms begin to arrange themselves in a defined and periodic manner which later
defines the crystal microstructure. Crystal size and shape are macroscopic
properties of
the crystal resulting from the internal crystal lattice structure.
[00148] After the nucleation begins, crystal growth also starts and both
nucleation and
crystal growth may occur simultaneously as long as supersaturation exists. The
rate of
nucleation and growth is determined by the existing supersaturation in the
solution and
either nucleation or growth occurs over the other depending on the
supersaturation
state. It is critical to define the concentrations of the reactants required
accordingly in
order to tailor the crystal size and shape. If nucleation dominates over
growth, finer
crystal size will be obtained. The nucleation step is a very critical step and
the
conditions of the reactions at this initial step define the crystal obtained.
By definition,
nucleation is an initial phase change in a small area such as crystal forming
from a
liquid solution. It is a consequence of rapid local fluctuations on a
molecular scale in a
homogeneous phase that is in a state of metastable equilibrium. Total
nucleation is the
32
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
sum effect of two categories of nucleation ¨ primary and secondary. In primary
nucleation, crystals are formed where no crystals are present as initiators.
Secondary
nucleation occurs when crystals are present to start the nucleation process.
It is this
consideration of the significance of the initial nucleation step that forms
the basis for the
CPF method.
[00149] In the CPF method, the reactants are dissolved in a solution
preferably at
ambient temperature or, if needed, at a slightly elevated temperature but
preferably not
more than 100 C. Selection of inexpensive raw materials and the proper
solvent are
important aspects of this invention. The purity of the starting materials are
also
important since this will affect the purity of the final product which may
need specified
purity levels required for its performance specifications. As such, low cost
starting
materials which can be purified during the preparation process without
significantly
increasing the cost of processing must be taken into consideration.
[00150] CPF uses conventional equipment to intimately mix reactants and
preferably
includes a highly agitated mixture preferably with bubbling of gas,
particularly, when
reactant gas is advantageous.
[00151] It is preferred that the gas be introduced directly into the solution
without limit
to the method of introduction. The gas can be introduced into the solution
within the
reactor by having several gas diffusers, such as tubes, located on the side of
the
reactor, wherein the tubes have holes for the exit of the gas. Another
configuration is to
have a double wall reactor such that the gas passes through the interior wall
of the
reactor. The bottom of the reactor can also have entry ports for the gas. The
gas can
also be introduced through the agitator shaft, creating the bubbles upon
exiting. Several
33
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
other configurations are possible and the descriptions of these arrangements
given
herein are not limited to these.
[00152] In one embodiment an aerator can be used as a gas diffuser. Gas
diffusing
aerators can be incorporated into the reactor. Ceramic diffusing aerators
which are
either tube or dome-shaped are particularly suitable for demonstration of the
invention.
The pore structures of ceramic bubble diffusers can produce relatively fine
small
bubbles resulting in an extremely high gas to liquid interface per cubic feet
per minute
(cfm) of gas supplied. A ratio of high gas to liquid interface coupled with an
increase in
contact time due to the slower rate of the fine bubbles can provide for a
higher transfer
rates. The porosity of the ceramic is a key factor in the formation of the
bubble and
significantly contributes to the nucleation process. While not limited thereto
for most
configurations a gas flow rate of at least one liter of gas per liter of
solution per minute is
suitable for demonstration of the invention.
[00153] A ceramic tube gas diffuser on the sides of the reactor wall is
particularly
suitable for demonstration of the invention. Several of these tubes may be
placed in
different positions, preferably equidistant from each other, to more uniformly
distribute
gas throughout the reactor. The gas is preferably introduced into the diffuser
within the
reactor through a fitting connected to the header assembly which slightly
pressurizes
the chamber of the tube. As the gas permeates through the ceramic diffuser
body, fine
bubbles may start to form by the porous structure of the material and the
surface
tension of the liquid on the exterior of the ceramic tube. Once the surface
tension is
overcome, a minute bubble is formed. This small bubble then rises through the
liquid
34
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
forming an interface for transfer between gas and liquid before reaching the
surface of
the liquid level.
[00154] A dome-shaped diffuser can be placed at the bottom of the reactor or
on the
sides of the reactor. With dome shaped diffusers a plume of gas bubbles is
typically
created which is constantly rising to the surface from the bottom providing a
large
reactive surface.
[00155] A membrane diffuser which closes when gas flow is not enough to
overcome
the surface tension is suitable for demonstration of the invention. This is
useful to
prevent any product powder from being lost into the diffuser.
[00156] In order to have higher gas efficiencies and utilization, it is
preferred to reduce
the gas flow and pressure and expend less pumping energy. A diffuser can be
configured such that for the same volume of gas, smaller bubbles are formed
with
higher surface area than if fewer larger bubbles are formed. The larger
surface area
means that the gas dissolves faster in the liquid. This is advantageous in
solutions
wherein the gas is also used to solubilize the reactant by increasing its
solubility in the
solution.
[00157] Nozzles, preferably one way nozzles, can be used to introduce gas into
the
solution reactor. The gas can be delivered using a pump and the flow rate
should be
controlled such that the desired bubbles and bubble rates are achieved. A jet
nozzle
diffuser, preferably on at least one of the sides or bottom of the reactor, is
suitable for
demonstration of the invention.
[00158] The rate of gas introduction is preferably sufficient to increase the
volume of
the solution by at least 5% excluding the action of the agitator. In most
circumstances
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
at least about one liter of gas per liter of solution per minute is sufficient
to demonstrate
the invention. It is preferable to recycle the gas back into the reactor.
[00159] Transfer of the added solution into the bulk solution is preferably
done using a
tube attached to a pump connecting the solution to be transferred to the
reactor. The
tube into the reactor is preferably a tube with a single orifice or several
orifices of a
chosen predetermined internal diameter such that the diameter size can deliver
a
stream of the added solution at a given rate. Atomizers with fine nozzles are
suitable for
delivering the added solution into the reactor. The tip of this transfer tube
can comprise
a showerhead thereby providing several streams of the added solution
simultaneously.
In large scale production, the rate of transfer is a time factor so the
transfer rate should
be sufficiently rapid to produce the right size desired.
[00160] The agitator can be equipped with several propellers of different
configurations, each set comprising one or more propellers placed at an angle
to each
other or on the same plane. Furthermore, the mixer may have one or more sets
of these
propellers. The objective is to create sufficient turbulence for adequate
solution
turnover. Straight paddles or angled paddles are suitable. The dimensions and
designs
of these paddles determine the type of flow of the solution and the direction
of the flow.
A speed of at least about 100 rotations per minute (rpm's) is suitable for
demonstration
of the invention.
[00161] The rate of transfer of added solution to the bulk solution has a
kinetic effect
on the rate of nucleation. A preferred method is to have a fine transfer
stream to control
the local concentration of the reactants which influences nucleation and the
rate of
nucleation over the rate of crystal growth. For smaller size powder, a slower
transfer
36
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
rate will yield finer powders. The right conditions of the competing
nucleation and
growth must be determined by the final powder characteristics desired. The
temperature
of reaction is preferably ambient or under mild temperatures if needed.
[00162] Special nanostructures are preformed which are carried over to the
final
product thus enhancing the performance of the material in the desired
application. For
the purposes of the present invention nanostructures are defined as structures
having
an average size of 100 to 300 nm primary particles.
[00163] Neither surfactants nor emulsifiers are necessary. In fact, it is
preferable that
surfactants and emulsifiers are not used since they may inhibit drying.
[00164] Size control can be done by concentration of the solutions, flow rate
of the
gas or transfer rate of added solution to the bulk solution.
[00165] No repetitive and cumbersome milling and classification steps are
used.
[00166] Reduced calcination time can be achieved and repetitive calcinations
are
typically not required.
[00167] Reaction temperature is ambient. If need for solubilization,
temperature is
increased but preferably not more than 100 C.
[00168] Tailored physical properties of the powder such as surface area,
porosity, tap
density, and particle size can be carefully controlled by selecting the
reaction conditions
and the starting materials.
[00169] The process is easily scalable for large scale manufacturing using
presently
available equipment and/or innovations of the present industrial equipment.
EXAMPLES
Electrode preparations:
37
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00170] The composite electrodes were prepared by mixing the active material
with
wt% conductive carbon black, as a conductive additive, 5 wt% polyvinylidene
fluoride
(PVDF), as a binder, dissolved in N-methyl-2-pyrrolidinone (NMP) solvent. The
slurry
was cast on graphite-coated aluminum foil and dried overnight at 60 C under
vacuum.
Electrode disks with an area of 1.54 cm2 were cut form the electrode sheets
with a
typical loading of 4 mg.cm-2.
Coin cell assembly:
[00171] Coin cells were assembled in an argon-filled glovebox. Lithium foil
(340 pm)
was used as counter and reference electrodes in half-cells, and commercial
Li4Ti6012
(LTO) composite electrodes were used as counter and reference electrodes in
full-cells.
1 M LiPF6 in 7:3 (vol%) ethylene carbonate (EC):diethylene carbonate (DEC) was
used
as the electrolyte. The electrodes were separated by one or two 25pm thick
sheets of
Celgard membranes in half-cells, and one sheet of Celgard membrane full-
cells.
Cycling protocol:
[00172] The spinel cathode cells were galvanostatically cycled in the voltage
range of
3.5 V ¨4.9 Vat various C-rates (1C rate equivalent to 146 mAg-1) at 25 C,
using an
Arbin Instrument battery tester (model number BT 2000). A constant voltage
charging
step at 4.9 V for 10 minutes was applied to the cells at the end of 1C or
higher rate
galvanostatic charging steps. The rock-salt NMC cells were galvanostatically
cycled in
the voltage range of 2.7 V ¨4.35 V at various C-rates (1C rate equivalent to
200 mAg-1)
at 25 C. A constant voltage charging step of 4.35 V for 10 minutes was
applied to the
cells at the end of 1C or higher rate galvanostatic charging step.
Example 1:
38
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00173] An SEM analysis of spray dried mixed oxalate precursor and calcined
material from the production of LiNi0.5Mn1.504 cathode material are both
crystalline and
the use of transition metal acetate and carbonate feedstocks provide a similar
material
morphology as illustrated in Fig. 5.
Example 2:
[00174] Fig. 6 shows the XRD patterns of manganese oxalate hydrates
precipitated
from the reaction of manganese carbonate and oxalic acid (5% excess by mole)
in
water for 6 hours (a) at room temperature in air (b) at room temperature with
nitrogen
bubbling (c) at room temperature with carbon dioxide bubbling (d) at water
reflux
temperature in air and (e) at room temperature in air with water content of 10
times of
those in experiments (a-d). The XRD patterns of the materials precipitated in
experiments (a-c) matches with that of manganese oxalate dihydrate with space
group
C2/c. N2 and CO2 gas bubbling have slightly affected the crystallinity of the
material.
The reaction at water reflux temperature (b) has produced two different
manganese
oxalate dihydrate phases; one in C2/c space group and one in P212121 space
group. A
decrease in concentration of the reactants to 1/10th of that in experiments (a-
d) resulted
in formations of catena-Poly[piaquamanganese(11)]-p-oxalato]monohydrate],
which has
a one-dimensional chain structure with space group Pcca. These experiments
demonstrate the significant effect of reaction conditions, such as
temperature,
concentration, and atmosphere, on the precipitated product of the reaction of
manganese carbonate and oxalic acid in water.
Example 3:
39
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00175] A particular problem with LiNi0.5Mn1.504 spinels is the phenomenon
referred to
as the 4V plateau wherein the voltage drops from 4.7 V to 4.0 V at the end of
discharge
as illustrated in Fig. 7. The plateau is believed to be the result of Mn3+
being formed
due to oxygen loss during firing in air. In the results with the prior art
process illustrated
in Fig. 7 an ordered precursor to the oxide was formed as a precipitate
comprising
nickel carbonate and manganese carbonate, with stoichiometric lithium acetate,
the
precursor to the oxide was calcined providing a spinel of LiNi0.43Mn1.5704
wherein the
Mn:Ni ratio was 3.70. The charge capacity as a function of voltage was
measured
resulting in the significant 4 volt plateau illustrated in Fig. 7.
[00176] In Inventive A the oxalate salts were formed from transition metal
acetates
resulting in a significant reduction in the 4-volt plateau as illustrated in
Fig. 7. In
Inventive A, an ordered precursor to the oxide was formed from lithium
carbonate,
nickel acetate and manganese acetate with oxalic acid digestion in a process
referred to
in Fig. 7. The precursor to the oxide was then calcined providing a spinel of
LiNi0.48Mn1.5204 wherein the Mn:Ni ratio was 3.13. The discharge capacity as a
function
of voltage was measured resulting in a significant reduction of the 4 volt
plateau as
illustrated in Fig. 7.
[00177] In Inventive B metal carbonates are used as the feedstock, with
oxalate
digestion of the carbonates resulting in the 4-volt plateau being essentially
eliminated
particularly with the use of a slight excess of nickel wherein the ratio of Mn
to Ni is no
more than 3, preferably at least 2.33 to less than 3 and most preferably 2.64
to less
than 3. An ordered precursor to the oxide was formed from lithium carbonate,
nickel
carbonate and manganese carbonate with oxalic acid digestion in a process
referred to
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
in Fig. 7 as the optimized process. The precursor to the oxide was calcined
providing a
spinel of LiNi0.51Mn1.4904wherein the Mn:Ni ratio was 2.90. The discharge
capacity as a
function of voltage was measured resulting in almost complete elimination of
the 4 volt
plateau as illustrated in Fig. 7.
Example 4:
[00178] A precursor for a high voltage spinel having a formula of
LiNi0.5Mn1.504 was
synthesized using lithium carbonate, nickel carbonate, manganese carbonate,
and
oxalic acid. 820.0 g of H2C204.2H20 was added to 2.0 L of water in a chemical
reactor
vessel at temperature of about 40 C. In a second vessel a carbonate mixture
slurry
was prepared comprising Li2CO3(96.1 g), NiCO3 (148.4 g), MnCO3 (431.1 g) in
1.2 L of
deionized water. The carbonate mixture slurry was pumped into the chemical
reactor
vessel at a rate of about 0.2 ¨ 0.3 L/h. The mixture within the reactor was
vigorously
mixed at 40 C in ambient atmosphere to form a slurry. The slurry was dried
using a
spray drier, producing the high voltage spinel precursor material. The X-ray
diffraction
(XRD) pattern is provided in Fig. 8 and a scanning electron microscopy (SEM)
image of
the dried powder is provided in Fig. 9. The XRD diffraction indicates a highly
ordered
crystalline lattice and the SEM demonstrates nanostructured crystalline
material.
Example 5:
[00179] A high voltage spinel having a formula LiNia5Mn1.504 was prepared from
the
precursor of Example 4. The precursor of Example 4 was placed in alumina
crucibles
and fired in a box furnace in air at 900 C for 15 h in ambient atmosphere. The
resulting
powder was analyzed by powder X-ray diffraction analysis resulting in the
diffraction
pattern provided in Fig. 10. The SEM provided in Fig. 11 illustrates that the
41
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
nanostructure of the precursor was largely maintained. The lattice parameter
of the
spinel structure was calculated to be 8.174(1) A. The electrochemical
performance of
the synthesized material was evaluated as the cathode in half cells versus
lithium metal
anodes and in full cells versus Li4Ti5012 (LTO) anodes. The voltage as a
function of
discharge capacity in a half cell at 0.1C is illustrated in Fig. 12. The
specific capacity as
a function of cycles at a 1C rate at 25 C in a half cell is illustrated in
Fig. 13. The
specific capacity at various discharge rates at 25 C in a half cell is
illustrated in Fig. 14.
The specific capacity at at 1C at 25 C in a full cell with a LTO anode is
illustrated in Fig.
15.
Example 6:
[00180] A high voltage spinel having a formula LiNia5Mn1.504 was prepared from
the
precursor of Example 4. The precursor material was placed in alumina boats and
fired
in a tube furnace under an oxygen flow of 50 cm3/m in. The firing procedure,
illustrated
in Fig. 16, included a pre-firing step at 350 C, firing at 900 C and slow
cooling to and
annealing at 650 C. Firing in oxygen in addition to slow cooling mitigates
the oxygen
deficiency and leads to a reduction in the 4V plateau commonly observed in
these
materials. The X-ray diffraction of the obtained powder is provided in Fig. 17
and,
based thereon, the lattice parameter of the Spinel structure was calculated to
be
8.168(1) A. The electrochemical performance of the synthesized material was
evaluated
as the cathode in half cells versus lithium metal anodes. The voltage profile
obtained at
a discharge rate of 0.1C at 25 C in a half cell is illustrated in Fig. 18. A
particular
feature is the absence of a 4V voltage plateau commonly observed in these
materials.
The specific capacity obtained at a 1C cycle rate at 25 C in a half cell is
illustrated in
42
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
Fig. 19. The specific capacity obtained at various discharge rates at 25 C in
a half cell
is illustrated in Fig. 20.
Example 7:
[00181] The precursor material of Example 4 was placed in alumina crucibles
and
fired in a box furnace in ambient atmosphere using the firing procedure
illustrated in Fig.
16. The X-ray diffraction pattern of the resulting powder is provided in Fig.
21 and the
lattice parameter of the spinel structure was calculated 8.169(1) A. The
electrochemical
performance of the synthesized material was evaluated as the cathode in half
cells
versus lithium metal anodes. The voltage as a function of discharge capacity
at a
discharge rate of 0.1C at 25 C in a half cell is illustrated in Fig. 22. The
specific
capacity obtained at a 1C discharge rate at 25 C in a half cell is illustrated
in Fig. 23.
Example 8:
[00182] A precursor to a high voltage spinel having formula LiNi0.5Mn1.504 was
synthesized using 8.62 g of MnCO3 (Alfa; Particle Size: 1-3 pm), 2.97 g of
NiCO3 (Alfa;
Anhydrous), and 1.92 g of lithium carbonate as the starting materials. 16.4 g
of oxalic
acid dihydrate (H2C204.2H20) was used as the chelating agent. The metal
carbonates
were mixed with 20 m L of DI water to form a slurry in one beaker and the acid
was
added to 40 m L of DI water inside a separate beaker. The oxalic acid slurry
was then
heated to 40 C and the carbonate slurry was added to the acid solution at a
rate of 8.9
m L/hr to form the precursor. The precursor was dried using a spray drier. The
dried
precursor was fired in an alumina crucible at 900 C for 15 hours in ambient
atmosphere. The voltage as a function of discharge measured at a discharge
rate of
0.1C at 25 C in a half cell is illustrated in Fig. 24.
43
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
Example 9:
[00183] A precursor to a high voltage spinel with formula LiNi0.5Mn1.504 was
synthesized similarly to Example 8 except a MnCO3 with a larger particle size
was
utilized (Sigma; Particle Size: 74 pm). The precursor was dried and fired
similarly to
Example 8. The voltage as a function of discharge measured at a discharge rate
of
0.1C at 25 C in a half cell is illustrated in Fig. 25.
Example 10:
[00184] A precursor to a high voltage Spinel LiNi0.5Mn1.504 was synthesized
using
8.62 g of MnCO3 (Sigma; Particle Size: 7m), 2.97 g of NiCO3 (Alfa; Anhydrous),
and
1.92 g of lithium carbonate as the starting materials. 16.4 g of oxalic acid
dihydrate
(H2C204.2H20) was used as the chelating agent. The metal carbonates were mixed
with
80 mL of DI water to form a slurry in one beaker and the acid was dissolved in
120 mL
of DI water inside a separate beaker. The carbonate slurry was added to the
oxalic acid
solution at ambient temperature of about 25 C at a rate of 16 mL/hr to form
the
precursor. The precursor was then dried using a spray drier. The dried
precursor was
fired in an alumina crucible at 900 C for 15 hours in ambient atmosphere. The
voltage
as a function of discharge measured at a discharge rate of 0.1C at 25 C in a
half cell is
illustrated in Fig. 26.
Example 11:
[00185] A precursor to a high voltage Spinel having formula LiNi0.5Mn1.504 was
synthesized similarly to Example 10 except less water was used in the
reaction: the
same amounts of metal carbonates were mixed with 12 mL of DI water and the
same
amount of oxalic acid was added to 28 mL of water. The carbonate slurry was
added to
44
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
the oxalic acid slurry at the rate of 3 mL/hr. The precursor was then dried
and fired
similarly to Example 7. The voltage as a function of discharge measured at a
discharge
rate of 0.1C at 25 C in a half cell is illustrated in Fig. 27. Example 11
demonstrates the
ability to form the precursor with very low amounts of added water, and in
some
embodiments no water is added, since water is provided by digestion and the
waters of
hydration of the starting materials may be sufficient to initiate and complete
the reaction.
Example 12:
[00186] A precursor to a high voltage spinel having formula LiNi0.5Mn1.504 was
synthesized similarly to Example 11 except a basic nickel carbonate (Sigma;
NiCO3.2Ni(OH)2.xH20), source was used. The precursor was then dried and fired
similarly to Example 11. The voltage as a function of discharge measured at a
discharge rate of 0.1C at 25 C in a half cell is illustrated in Fig. 28.
Example 13:
[00187] A precursor to a high voltage spinel with formula LiNi0.5Mn1.504 was
synthesized using 8.62 g of MnCO3 (Sigma; Particle Size: 74 pm), 2.97 g of
NiCO3
(Alfa; Anhydrous), and 1.92 g of lithium carbonate as the starting materials.
16.4 g of
oxalic acid dihydrate (H2C204.2H20) was used as the chelating agent. The metal
carbonates were mixed with 80 mL of DI water to form a slurry in one beaker
and the
acid was dissolved in 160 mL of DI water inside a separate beaker. The beaker
with the
dissolved oxalic acid was then placed inside an ice bath to maintain a
temperature of
about 5 C. The carbonate slurry was added to the oxalic acid solution at a
rate of 23
mL/hr. An XRD pattern of the dried precursor is provided in Fig. 29.
Example 14:
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00188] A precursor to a high voltage spinel having formula LiNi0.5Mn1.504
precursor
was synthesized similarly to Example 13 except the synthesis was carried out
at the
boiling point of water (100 C). A reflux condenser was used to maintain the
water level
of the reaction. An XRD pattern of the dried precursor is provided in Fig. 30.
Example 15:
[00189] A precursor to a spinel having formula LiMn204 was synthesized using
lithium
carbonate, manganese carbonate, and oxalic acid as starting materials. 16.39 g
of
H2C204.2H20 was added to 40 ml of water in a beaker. In a second beaker,
Li2CO3
(1.85 g) and MnCO3 (11.49 g) were mixed in 24 ml of deionized water. The
carbonate
mixture slurry was pumped into the oxalic acid slurry with a rate of 0.01
L/Hr. The
mixture within the reactor was mixed at ambient temperature. The resulting
slurry was
dried by evaporating, producing the precursor to the LiMn204. The XRD pattern
is
provided in Fig. 31.
[00190] The precursor material was fired in a box furnace in air at 350 C for
lh and
then 850 C for 5h. The X-ray diffraction pattern and scanning electron
microscopy
image of the fired material are shown in Figs. 32 and 33, respectively.
Example 16:
[00191] A precursor to a spinel of formula LiMn1.9M0.104 (M: Mn, Al, Ni) was
synthesized using metal carbonates and oxalic acid, in the amounts shown in
Table 1 .
[00192] The starting materials of each composition was mixed in 32 ml of
deionized
water for 6h at ambient temperature. The resulting slurries were dried by
evaporation.
The X-ray diffraction patterns shown in Fig. 34 show that manganese oxalate
dihydrate
(Sample A), a precursor to LiMn204, and the precursor to LiMn1.9A10.104
(Sample B)
46
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
crystalized in an orthorhombic space group (P212121). The
LiMn1.9Ni0.104(Sample C)
crystallized in a monoclinic space group (C2/c).
Table 1:
Sample Li2CO3 MnCO3 Al(OH)3 NiCO3 H2C204.2H20
A 0.961 g 5.745g 0 0 8.195g
0.961 g 5.465g 0.195g 0 8.195g
0.961 g 5.465g 0 0.297g 8.195g
Example 17:
[00193] A precursor to NMC 111 having formula LiNi0.333Mn0.333C00.33302 was
prepared
from 3.88 g Li2CO3, 3.79 g NiCO3, 3.92 g MnCO3, 3.93 g CoCO3 and 19.23 g of
H2C204.2H20 dispersed in 240 mL of deionized water in a round-bottom flask.
The
mixture was heated under reflux for 6.5 hour and allowed to cool down. The
final
mixture had a solids content of approximately 13 %. The powder was obtained by
spray
drying to obtain the precursor with the formula
LiNi0.333Mn0.333Coo.333(C204)1.6. The
precursor was heated at 110 C for 1 h and calcined at 800 C for 7.5 h under
air in a
box furnace to obtain NMC 111. An SEM of the precursor is provided in Fig. 36.
The
XRD pattern of the calcined powder is provided in Fig. 35 and the SEM of the
calcined
powder is provided in Fig. 37 wherein the nanostructure of the precursor is
shown to be
largely maintained. The discharge capacity as a function of cycles is
illustrated in Fig.
38.
Example 18:
[00194] A precursor to NMC 622 having formula LiNio.6Mno.2Coo.202 was prepared
from 39 g Li2CO3, 71 g NiCO3, 23 g MnCO3, and 24 g CoCO3 dispersed in 200 mL
of
47
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
deionized water in a beaker. The mixture of carbonates was pumped into a
separate
beaker containing 201 g of H2C204.2H20 in 400 mL of deionized water at a rate
of 0.38
moles of carbonates per hour. The reaction mixture was then stirred for 1 h.
The final
mixture, having a solids content of approximately 20%, was spray dried to
obtain the
precursor with the formula LiNi0.6Mn0.2Coo.2(C204)1.5. An XRD pattern of the
precursor is
provided in Fig. 39 and the SEM is provided in Fig. 41. The precursor was
heated at
110 C for 1 h and calcined at 800 C for 7.5 h under air in a box furnace to
obtain NMC
622 with an XRD pattern illustrated in Fig. 40 and an SEM of Fig. 42. The SEM
demonstrates that the ordered nanostructure lattice of the precursor is
substantially
maintained in the calcined powder. The discharge capacity of a half cell at 25
C at 1C
as a function of cycle number is shown in Fig. 43. Fig. 44 shows the initial
charge and
discharge voltage profiles as a function of capacity at 0.1C.
Example 19:
[00195] A precursor for NMC 811 having formula LiNi0.8Mno.iCoo.102 was
prepared
from 39 g Li2CO3, 95 g NiCO3, 12 g MnCO3, and 12 g CoCO3 dispersed in 200 mL
of
deionized water in a beaker. The mixture was pumped into a separate beaker
containing 201 g of H2C204.2H20 in 400 mL of deionized water at a rate of 0.38
moles
of carbonates per hour. The reaction mixture was then stirred for 1 h. The
final mixture
having a solids content of approximately 20% was spray dried to obtain the
precursor
with the formula LiNi0.8Mno.i Coo.i (C204)1.5. The precursor was heated at 600
C for 5 h
under air in a box furnace, heated at 125 C for 1 h under oxygen flow, and
calcined at
830 C for 15 h under oxygen flow in a tube furnace to obtain NMC 811. The XRD
pattern of the NMC 811 oxide is provided in Fig. 45. The discharge capacity as
a
48
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
function of cycles is provided in Fig. 46 and the voltage profile as function
of capacity is
illustrated in Fig. 47. The NMC 811 was heated at 125 C for 1 h and calcined
at 830
C for 15 h under oxygen flow in a tube furnace to form refired NMC 811. The
XRD
pattern of the refired XRD is provided in Fig. 48 and the SEM is provided in
Fig. 49.
The discharge capacity is provided in Fig. 50 wherein the solid curve
represents the
average capacity and the error bars represent the maximum and minimum
capacities
for a series of samples.
Example 20:
[00196] A precursor for NCA with formula LiNio.8Coo.16Alo.0602 was prepared
from 8 g
Li2CO3, 19 g NiCO3, 2g Al(OH)(CH3C00)2 and 4 g CoCO3 dispersed in 40 m L of
deionized water in a beaker. The mixture was pumped into a separate beaker
containing 40 g of H2C204.2H20 in 80 m L of deionized water at a rate of 0.08
moles of
carbonates per hour. The reaction mixture was then stirred for 1 h. The final
mixture
having a solids content of approximately 20% was spray dried to obtain the
precursor
with the formula LiNio.8Coo.16Alo.0602. The precursor was heated at 125 C for
1 h and
then calcined at 830 C for 15 h under oxygen flow in a tube furnace to obtain
NCA. The
XRD pattern is provided in Fig. 51 and an SEM is provided in Fig. 52 wherein
the
layered nanostructure originating in the precursor is readily observable.
Example 21:
[00197] NMC 622 having overall formula LiNio.6Mno.2Coo.202 was prepared with a
step-wise concentration gradient of transition metals from the central
portion, or core, to
the exterior. The precursor was prepared from 3.9 g Li2CO3, 9.5 g NiCO3, 1.2 g
MnCO3,
and 1.2 g CoCO3 dispersed in 10 mL of deionized water in a beaker. The mixture
is
49
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
pumped into a separate beaker containing 40.4 g of H2C204.2H20 in 80 mL of
deionized
water to form the core precursor. Subsequently, a mixture comprising 1.0 g
Li2CO3, 1.8
g NiCO3, 0.6 g MnCO3, and 0.6 g CoCO3 dispersed in 5 mL of deionized water was
pumped into the reaction mixture to form a first shell of precursor around the
core. An
additional mixture comprising 2.9 g Li2CO3, 3.0 g NiCO3, 2.9 g MnCO3, and 3.0
g
CoCO3 was dispersed in 10 mL of deionized water and pumped into the reaction
mixture to form a third ratio in a second shell around the first shell. The
addition rates
were kept constant at 15 mL per hour for each solution. The reaction mixture
was then
stirred for 1 h and spray dried to obtain the precursor with the overall
formula
LiNia6Mn0.2Coo.2(C204)1.5. The precursor was then heated at 110 C for 1 hand
calcined
at 800 C for 7.5 h under air in a box furnace to obtain gradient NMC 622 with
a nickel
rich core NMC 811 core having a formula of LiNi0.8Mno.1Coo.102, a first shell
of NMC 622
having a formula of LiNi0.6Mn0.2Co0.202 representing the bulk of the volume,
and an
outer NMC 111 shell having the form LiNi0.333Mn0.333Coo.33302. The invention
thereby
allows the surface characteristics to be different from the bulk. The XRD
pattern for the
step-wise NMC is provided in Fig. 53 and the SEM is provided in Fig. 54. The
discharge capacity as a function of cycles is provided in Fig. 55. A
comparative
illustration of discharge capacity for NMC 622 (Example 15), NMC 811 (Example
16),
NMC 811 fired twice (Example 16), NCA (Example 17) and NMC gradient (Example
18)
is provided in Fig. 56 and normalized in Fig. 57.
Example 22: Preparation of coated spinel
[00198] A coated spinel was formed having a niobiate coating on the surface.
The
precursor was prepared by adding 16.39 g of H2C204.2H20 to 40 mL of water in a
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
beaker. In a second beaker, Li2CO3 (1.92 g), NiCO3 (2.97 g), MnCO3 (8.62 g)
were
mixed in 24 mL of deionized water. The carbonate mixture slurry was slowly
added to
the oxalic acid slurry beaker (3 m L every 20 minutes) and mixed. The slurry
was mixed
at room temperature in ambient atmosphere overnight. The raw materials for
LiNb03,
0.816 g of Nb(HC204)5.xH20 (x=6.35 estimated from TGA) and 0.046 g of Li2CO3,
were
added to the slurry the next day. After 3 hours of mixing, the slurry was
dried by a spray
dryer. The precursor was fired for 5h, followed by an annealing step at 750 C
for 24 h.
[00199] The XRD pattern of the fired material, Fig. 58, shows the peaks of
spinel
LNMO as the main phase and the peaks of LiNb03 as the second phase. In Fig. 58
the
XRD pattern of LNMO with a lithium niobite coated is illustrated wherein the
second
panel shows the lithium niobite peaks expanded.
[00200] The SEM images of the material in Fig. 59 show particle sizes in
the range
of 500 nm - 2 pm which is smaller than the other spinel materials possibly due
to its
lower synthesis temperature. The images show some speckles on the surface of
the
particles. These speckles are probably not related to LiNb03 because a
pristine spinel
sample analyzed the same day by SEM showed a similar feature. SEM images do
not
show any clear evidence of separate LiNb03 particles, suggesting a coating on
the
spinel particles.
[00201] Scanning Transmission Electron Microscope (STEM) analyses were
carried
out on individual secondary agglomerates sampled by drop-casting a propyl
alcohol
(PrOH) suspension of the fired material onto a 200 mesh Cu transmission
electron
microscope (TEM) grid with a carbon-impregnated formvar support. High-Angle
Annular Dark Field (HAADF) image and Energy Dispersive Xray (EDX) map of Mn
and
51
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
Nb of one agglomerate showed separate primary crystallites of LiNb03 which
were
clearly visible. While crystallinity of the separated LiNb03 crystals was
confirmed via
High-Resolution TEM (HRTEM) the large size of the particles made it difficult
to confirm
the presence, crystallinity and thickness of any LiNb03 coating present.
[00202] In addition, quantification was done at high magnification for
regions in two
separate agglomerates. EDX maps showed a distinct outline of Nb around the
primary
particles along with an even, though lower intensity, distribution of Nb over
the bulk of
the particle. This is indicative of a very thin, though even LiNb03 coating.
It is not
possible to determine whether the Nb distribution in the sample bulk
corresponds solely
to surface Nb, or a doping of Nb throughout the bulk, however the peak
concentration
on the borders suggests the majority of the Nb is present on the surface and
the metal
in the surface is at least 95 wt% niobium. Overall, 5 regions over two
separate
agglomerates were sampled, yielding similar results.
[00203] The LiNb03-coated LNMO material was evaluated as the cathode
material
in three half-cells, with Li as the anode and 1 M LiPF6 in 7:3 (vol%) ethylene
carbonate
(EC):diethylene carbonate (DEC) was used as the electrolyte. The cells were
cycled
within the voltage range of 3.5 - 4.9 V at 0.1C for 1 cycle and at 1C for the
next ones at
55 C. shows the average specific capacity over 100 cycles. This material
shows
improved capacity retention at 55 C, Fig. 60, over the baseline material,
which usually
fails when cycled above 50 cycles at the 1C rate. This improved performance is
probably due to the protection of LNMO particles from reaction with
electrolyte at
elevated temperature due to the coating layer.
Comparative Example Cl
52
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00204] A precursor for NMC 811 having formula LiNi0.8Mno.iCoo.102 was
prepared
from 39 g Li2CO3, 95 g NiCO3, 12 g MnCO3, and 12 g CoCO3 dispersed in 200 mL
of
deionized water in a beaker. The mixture was pumped into a separate beaker
containing 201 g of H2C204.2H20 in 400 mL of deionized water at a rate of 0.38
moles
of carbonates per hour. The reaction mixture was then stirred for 1 h. The
final mixture
having a solids content of approximately 20% was spray dried to obtain the
precursor
with the formula LiNi0.8Mno.i Coo.i (C204)1.5. The precursor was heated at 600
C for 5 h
under air in a box furnace, heated at 125 C for 1 h under oxygen flow, and
calcined at
830 C for 15 h under oxygen flow in a tube furnace to obtain NMC 811. The NMC
811
was heated at 125 C for 1 h and calcined at 830 C for 15 h under oxygen flow
in a
tube furnace to form refired NMC 811 referred to herein as "Pristine NMC811".
Inventive Example Cl:
[00205] A precursor to NMC 811 having formula LiNi0.8Mno.iCoo.102 was prepared
by
adding 0.267 moles of nickel(11) carbonate hydrate (Alfa Aesar, 99.5% metal
basis), 0.1
mole of cobalt(II) carbonate (Alfa Aesar, 99% metal basis) and 0.1 mole of
manganese(II) carbonate (Sigma Aldrich > 99.9% metal basis) and 0.525 mole of
lithium carbonate (Alfa Aesar, 99%) to 200 mL deionized water with stirring
for 30
minutes to prepare a carbonate slurry. In a separate beaker, 1.617 moles of
oxalic acid
dihydrate was added to 400 mL of deionized water with stirring for 30 minutes.
The
carbonate slurry was added dropwise to the oxalic acid dihydrate mixture over
5 hours
an stirred for an additional 18 hours to prepare the oxalate slurry.
[00206] The coating solution was prepared by adding 0.005 moles of niobium(V)
oxalate hydrate (Alfa Aeser) with stirring over-night. The coating solution
was added to
53
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
the oxalate slurry followed by stirring for an additional 2 hours prior to
spray drying. The
resulting powder was fired at 830 C for 15 hours in a tube furnace under
oxygen flow.
The powder was ground to a sieve size of <45 i_tm and vacuum sealed in an
aluminum
bag. The resulting powder is referred to herein as 1-pot coated NMC811.
[00207] Inventive Example Cl and Comparative Example Cl would be characterized
for electrical properties. Inventive Example Cl would show an improved
discharge
capacity after repeated cycling as illustrated graphically in representative
Fig. 61 with
the expected normalized discharge capacity illustrated graphically in
representative Fig.
62. Expected improvements in the rate capability of the inventive example are
illustrated in representative Fig. 63.
Comparative Example C2
[00208] A precursor to NMC 622 having formula LiNio.6Mno.2Coo.202 was prepared
from 39 g Li2CO3, 71 g NiCO3, 23 g MnCO3, and 24 g CoCO3 dispersed in 200 mL
of
deionized water in a beaker. The mixture of carbonates was pumped into a
separate
beaker containing 201 g of H2C204.2H20 in 400 m L of deionized water at a rate
of 0.38
moles of carbonates per hour. The reaction mixture was then stirred for 1 h.
The final
mixture, having a solids content of approximately 20%, was spray dried to
obtain the
precursor with the formula LiNi0.6Mn0.2Coo.2(C204)1.5. The precursor was
heated at 110
C for 1 h and calcined at 800 C for 7.5 h under air in a box furnace to
obtain NMC
622.
Inventive Example C2:
[00209] A precursor to NMC 622 having formula LiNio.6Mno.2Coo.202 would be
prepared from 39 g Li2CO3, 71 g NiCO3, 23 g MnCO3, and 24 g CoCO3 dispersed in
200
54
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
M L of deionized water in a beaker. The mixture of carbonates would be pumped
into a
separate beaker containing 201 g of H2C204.2H20 in 400 mL of deionized water
at a
rate of 0.38 moles of carbonates per hour. The reaction mixture would then
stirred for 1
hour.
[00210] The coating would be prepared by adding 3.2g Niobium (V) oxalate
hydrate to
the reaction mixture and leaving stirring for an additional 2 hours. The final
mixture,
having a solids content of approximately 20%, would be spray dried to obtain
the
precursor. The precursor would be heated at 110 C for 1 h and calcined at 800
C for
7.5 h under air in a box furnace to obtain a one-pot coated NMC 622.
Comparative Example C3:
[00211] A precursor for NCA with formula LiNio.8Coo15Alo.0502 was prepared
from 39g
Li2CO3, 95g NiCO3, 8g Al(OH)(CH3C00)2, and 18g CoCO3 was dispersed in 200m1
deionized water in a beaker. This mixture was pumped into a separate beaker
containing 201g oxalic acid hydrate in 400m1 deionized water at a rate of 0.38
moles of
carbonates an hour. The reaction mixture was stirred for one hour. The
precursor was
heated at 125 C for 1 h and then calcined at 830 C for 15 h under oxygen
flow in a
tube furnace to obtain NCA.
Inventive Example C3:
[00212] A precursor for NCA with formula LiNio.8Coo15Alo.0502 was prepared
from 39g
Li2CO3, 95g NiCO3, 8g Al(OH)(CH3C00)2, and 18g CoCO3 dispersed in 200m1
deionized water in a beaker. This mixture was pumped into a separate beaker
containing 201g oxalic acid hydrate in 400m1 deionized water at a rate of 0.38
moles of
carbonates an hour and then stirred for 1 hour.
CA 03196927 2023-03-27
WO 2022/077105 PCT/CA2021/051436
[00213] The coating would be prepared by adding 3.2g Niobium (V) oxalate
hydrate to
the reaction mixture and leaving stirring for an additional 2 hours. The final
mixture,
having a solids content of approximately 20%, would be spray dried to obtain
the
precursor. The precursor would be heated at 125 C for 1 h and then calcined
at 830 C
for 15 h under oxygen flow in a tube furnace to obtain one-pot coated NCA.
[00214] The invention has been described with reference to the preferred
embodiments without limit thereto. One of skill in the art would realize
additional
embodiments and improvements which are not specifically set forth herein but
which
are within the scope of the invention as more specifically set forth in the
claims
appended hereto.
56