Note: Descriptions are shown in the official language in which they were submitted.
CA 02944898 2016-10-07
REDUCING MULTIVALENT-CATION NAPHTHENATES IN
BITUMEN THROUGH THE USE OF NANOPARTICLE OR SUB-NANO
PARTICLE ZEOLITES
Field
The invention relates generally to processing of bitumen ore.
Background
Extraction of bitumen from bitumen ore normally involves forming a water-
based slurry which permits the bitumen to be separated from sand, clay and
other particulates in the ore. The ore may contain naturally-occurring
naphthenic acids.
In some processes, the pH of the ore is elevated during processing by adding a
caustic compound into the slurry-forming water. This serves to increase the
.. extraction efficiency of bitumen. However, this also permits multivalent
cations
such as calcium that are present in the slurry from the slurrying water or
which
are liberated in the extraction process, to react with napthenic acids in the
bitumen, to thereby form calcium naphthenate or other multivalent-cation
naphthenate compounds. The calcium naphthenate in turn can reduce the
effectiveness of zeolite catalysts that are used in the downstream upgrading
of
bitumen.
For example, sodium hydroxide is commonly added to the ore-slurrying water in
order to raise the pH of the bitumen ore slurry. Partial saponification of
organic
components with the caustic increases the overall bitumen liberation and yield
but in so doing converts the natural naphthenic acids contained in the bitumen
to naphthenates. The multi-valent cations in the process water (such as
calcium
and magnesium) react to produce, particularly, calcium naphthenate. In
subsequent downstream bitumen purification steps that employ expensive
zeolite catalysts, this catalyst is poisoned by the calcium naphthenate,
greatly
increasing the cost of upgrading the bitumen.
1
Zeolites have known uses in oil sands processing. An example of a process for
separating bitumen from ore using zeolites is disclosed in Canadian Patent
2667933 by Edwin T. Sortwell (herein '933). '933 describes a process in which
zeolite particles are used to enhance the extraction of bitumen from oil sands
ores, such as those mined in the Canadian provinces of Alberta and
Saskatchewan. In one process, ore is slurried in hot water at the inlet of a
pipeline and the slurry is transported to a primary separating vessel where
the
bitumen is floated off. To simulate the pipeline process, zeolite particles
are
added to the dispersing water to disperse the components of the oil sands ore,
namely bitumen, clay and sand (and sometimes other organics), to more
completely liberate bitumen product from the other slurry components.
However, '933 does not describe any means to reduce calcium naphthenates in
the slurry or prevent its formation.
Zeolites can be naturally-occurring or synthetically produced. Examples of
naturally-occurring mineral zeolites include analcime, chabazite,
clinoptilolite,
heulandite, natrolite, phillipsite and stilbite. Zeolites may also be produced
synthetically. There are several types of synthetic zeolites that form by a
process
of slow crystallization of a silica-alumina gel in the presence of alkalis and
organic templates. One of the important processes used to carry out zeolite
synthesis is sol-gel processing. The product properties depend on reaction
mixture composition, pH of the system, operating temperature, pre-reaction
'seeding' time, reaction time as well as the templates used. In sol-gel
process,
other elements (metals, metal oxides) can be easily incorporated.
Zeolites consist of a three-dimensional crystal framework of SiO and A10-
tetrahedra. The tetrahedra are joined by the sharing of oxygen atoms, so that
the
ratio of oxygen atoms to the total of silicon and aluminum atoms is two.
Silicon is
tetravalent so that if two silicon/oxygen tetrahedra were joined (two silicon
atoms with four oxygen atoms), the resultant structure would be
electroneutral.
However, aluminum is trivalent, so that two joined tetrahedra made up of one
silicon atom, one aluminum atom, and four oxygen atoms would be
electronegative with one of the oxygens having only one of its charges
satisfied.
This deficiency must be satisfied with the inclusion of a cationic charge for
each
aluminum atom involved. This cation may be exchanged for another cation, e.g.,
2
CA 2944898 2018-08-01
CA 02944898 2016-10-07
sodium ions may be exchanged for calcium ions. The zeolite tetrahedra
structures are four-sided and also have four points. The oxygen atoms are
located
at each point, and the silicon or aluminum atoms are located at the center of
the
tetrahedron. It is at an oxygen atom at one of the aluminum bearing
tetrahedron
points where the included exchangeable ration is located.
Synthetic zeolites are widely used as catalysts in the petrochemical industry,
for
instance in fluid catalytic cracking and hydrocracking. Zeolites confine
molecules in small spaces, which causes changes in their structure and
reactivity.
Nagan U.S. Patent No. 6,190,561 (herein '561) describes a procedure for
synthetically producing a type A zeolite by reacting sodium aluminate and
either
sodium or potassium silicate, relatively inexpensive and commercially
available
chemicals. Both sodium silicate and sodium aluminate are available as bulk
liquids.
In Nagan '561, the disclosure of which may be referred to for further details,
there is also described a process using sodium or potassium zeolite
crystalloid
coagulants ("ZCC") for water clarification.
The aluminum and silica components that form the zeolites typically contain
excess caustic that can elevate the pH of a bitumen slurry, when a zeolite
solution
prepared synthetically from such compounds is added to the slurry.
Summary
We disclose herein a process for reducing the concentration of calcium (and
other multivalent-cation) naphthenate in a bitumen slurry that contains
bitumen ore, in order to improve the efficiency of downstream treatment of the
bitumen with various catalysts that may be otherwise inhibited by the presence
of calcium naphthenate.
It was the surprising discovery of the inventors that using zeolite particles
in the
aqueous phase of a bitumen ore/water slurry, is highly effective at preventing
the formation of unwanted multivalent-cation naphthenate compounds in
bitumen extracted by an aqueous slurry process. For this purpose, according to
3
CA 02944898 2016-10-07
one aspect, zeolite particles are introduced into water that is subsequently
used
to form the bitumen ore slurry. The zeolite particles sequester multivalent
cations in the slurry water so as to reduce or prevent the subsequent
formation
of multivalent naphthenates (such as calcium naphthenate) in the slurry.
In one aspect, we disclose a process for using zeolite particles for the
reduction
or prevention of the formation of multivalent-cation naphthenates such as
calcium naphthenate, in bitumen extracted from oil sands ore.
One aspect consists of a method of treating oil sands ore, wherein the ore
contains bitumen, particulate matter and napthenic acid. According to this
aspect, the method comprises the steps of combining zeolite particles with
water
to form a zeolite/water mixture and contacting the ore with the mixture to
form
a slurry containing said mixture, whereby the zeolite particles sequester at
least
a portion of multivalent cations present within the water to prevent or limit
the
formation of multivalent-cation naphthenate compounds within the slurry.
The method may involve adding zeolite particles to water to form a
water/zeolite
mixture, and then contacting the bitumen ore with said water/zeolite mixture
to
form a slurry.
The method may further include the step of adding a caustic compound such as
sodium hydroxide to the slurry water to increase bitumen extraction.
According to this method, a mixture of water, zeolite particles and the
caustic
compound is formed. The zeolite particles will then sequester multivalent
cations such as calcium that are present in the water mixture or which are
released into the water. The bitumen ore is then contacted by the water
mixture
to form a slurry that contains water, the caustic compound and sequestered
calcium. The resulting slurry may also contain residual zeolite particles that
are
able to sequester any additional multivalent cations that may be present in
the
slurry or which are released into the aqueous phase of the slurry subsequent
to
the slurry-forming step. In one aspect, a caustic compound is added to the
zeolite/water mixture before contacting the ore.
4
CA 02944898 2016-10-07
According to another aspect, excess caustic in the solution of zeolite
particles
(i.e. caustic generated by the excess caustic present in the solution after
formation of the zeolite particles from aluminum and silica starting
materials)
elevates the pH of the slurry, thereby minimizing the requirement to add any
additional caustic compound.
According to one aspect, the zeolite particles have an average diameter which
is
less than 4 nm, or less than 3 nm, or less than 2 nm or less than 1 urn.
According to a further aspect, the zeolite particles are in the range of 4 to
100
nm in average diameter.
The zeolite particles may be added to form a concentration of 500 ppm to 2400
ppm of zeolite active (w/w based on the weight of ore) within the slurry or,
according to a further aspect, a concentration of about 1600 ppm.
According to one aspect of the method described herein, multivalent cations
such as calcium and magnesium are sequestered from the slurry-forming water
and/or the bitumen slurry before these cations can react with napthenic acid
within the bitumen ore to thereby minimize the formation of multivalent-cation
naphthenates such as calcium naphthenate. According to a further aspect, an
excess of zeolite particles is added to the slurry and/or slurry-forming water
whereby the excess contains sufficient particles to sequester any multivalent
cations present in the water before forming the slurry as well as multivalent
cations that are released into the aqueous phase of the slurry, after the
slurry is
formed.
The use of zeolites in the nano-particle size range or in the sub-nano
particle size
range thus serves to prevent or limit the formation of calcium naphthenate
within the bitumen slurry which would otherwise occur upon addition of a base
if calcium ions are present. The treated bitumen product may then be contacted
with a catalyst such as a zeolite catalyst in the course of downstream
upgrading
of the bitumen. The sacrificial sequestering of calcium ions (and other
multivalent cations) by the zeolite particles is therefore used to protect the
downstream zeolite catalysts that in turn are used to upgrade bitumen quality.
Furthermore, according to another aspect, the increased pH of the extraction
5
CA 02944898 2016-10-07
process resulting from the caustic residual of the zeolite "solution" improves
extraction of bitumen, per se, i.e. without the addition of any additional
caustic
substance.
The zeolite particles sequester multivalent cations that are present in the
slurrying water or which are liberated from the ore, in particular calcium
ion,
thereby minimizing or preventing the formation of undesirable calcium
naphthenate within the bitumen. In effect, the nanoparticle or sub-nano
particle zeolite is a sacrificial protection of the expensive upgrading
zeolite
catalysts used downstream. The zeolite is produced by reacting sodium silicate
and sodium aluminate, both caustic solution products. This process is
described in detail in '933. Since there is caustic residual in the zeolite
"solution", the pH of the slurried bitumen in the pipeline is raised to
enhance
bitumen extraction while at the same time sequestering the calcium ion to
prevent formation of calcium naphthenate.
According to the above alternative, the excess caustic in the zeolite solution
elevates the pH of the mixture to thereby improve the extraction efficiency of
the
bitumen processing steps, while also sequestering multivalent cationic
compounds that are released into the aqueous phase.
Definitions
"Nanoparticles" refers to particles having an average size which is in the
range of
about 1-100 nm. Nanoparticles used in the present invention normally have an
average size of <iNM to about 4 nm.
Sub-nano particles refers to particles having an average diameter which is
less
than 1 nm.
"Zeolites" refers to a microporous aluminosilicate material. As used herein,
"zeolite" refers to a synthetically produced product unless otherwise
specified or
the context makes clear.
6
CA 02944898 2016-10-07
Brief description of the drawings
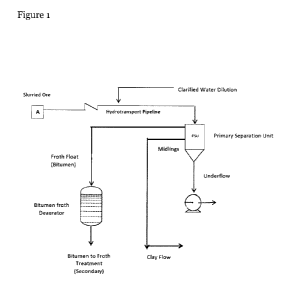
Figure 1 is a process flow diagram of an oil sands extraction process which
includes an embodiment of the present invention.
Figure 2 is a schematic view of a laboratory bitumen extraction unit ("BEU")
used to test an example of the invention.
Detailed description
A non-limiting description of selected embodiments of the invention is
described
herein.
One embodiment utilizes zeolite particles produced by the reaction of sodium
aluminate with either sodium silicate or potassium silicate, all in solution
form.
These inorganic reagents are commercially available in aqueous solution form.
Sodium aluminate is available as the product SAX 20TM from Kemira
Corporation. Sodium silicate is available in solution form as PQ "M" TM brand
from PQ Corporation. These reagents are diluted with water and reacted to form
a type A (ion exchange) zeolite, for example as described in Nagan '561, which
describes the use of zeolite particles of at least 4 nm in diameter. 4 nm is
generally recognized to be the particle size at which opalescence may be
observed. However, in the present embodiment the process may be modified to
produce zeolite particles that have an average diameter of less than 4 nm, and
optionally less than 3 nm, less than 2 nm and optionally less than 1 nm (i.e.
sub-
nanometre particles). Alternatively, the zeolite particles may be greater than
4
nm in average diameter, such as in the range of 4-100 nm.
It has been discovered that a functional dispersing zeolite can be formed as a
solution, in a nearly instantaneous reaction of aluminate and silicate. For
.. example, the reaction time may be up to fo seconds or between 2 and 5
seconds.
This greatly simplifies production of zeolite by reducing the control
parameters
needed for on-site production of zeolite from the two commercially available
reagents. Furthermore, it results in particles sizes in the sub-nano size
range,
namely less than 1 nm in diameter on average. The longer the reaction
duration,
7
CA 02944898 2016-10-07
the larger the particle size. In this fashion, particle sizes of between 1 and
4 nm
can also be produced, as well as sub-nanometre particles.
These rapidly-reacted zeolite particles sequester multivalent cations in a
similar
manner to the larger zeolite particles of 4 nm to loci nm described in Nagan.
Preferably, the zeolite is synthetically produced from the two ionic reactants
under controlled reaction and quenching procedures in order to control the
particle size as described herein.
The rate of the zeolite-formation reaction is dependent upon concentration and
temperature. In the manufacture of zeolites for water softening, relatively
high
concentrations of sodium aluminate and sodium silicate are used because the
goal is to produce strong, relatively large particle solids that can support
one
another in zeolite beds several feet high. These high concentrations instantly
produce a solid mass of sodium aluminum silicate (a zeolite), infused with
water.
According to the invention, the zeolite crystalloids offer a system of calcium
or
other multivalent cation "hooks" on their surfaces by virtue of having cation
exchange sites offered by the aluminum tetrahedra in the crystalloid lattice
structure.
In various embodiments, an oil sands deposit is mined to obtain bitumen-
containing ore. The ore typically contains bitumen which is adhered to and
mixed with sand and/or clay and in some cases certain other organic materials.
The mined ore is ground and combined with hot water to form a slurry. The
slurry is pumped through a pipeline that may be several kilometers long, in a
process that is called "hydrotransport" that transports the slurry to
downstream
processing whereby bitumen is floated off. During travel through the pipeline,
turbulence within the pipeline mixes and separates components of the ore. Just
before the slurry enters the primary separation unit, dilution water is added
to
the slurry to facilitate floatation.
Fig. 1 is a flow diagram of an oil sands extraction process according to an
embodiment of the invention. Point "A" in Figure 1 is the point at the start
of
hydrotransport pipeline where zeolite solution is added to heated water before
8
CA 02944898 2016-10-07
the heated water contacts the ore. For convenience, the zeolite/water mixture
will be referred to herein as a "solution" although in some cases with a
sufficiently large particle size the zeolite may be considered in a
dispersion. The
term "solution" as used herein refers generally to a classical solution as
well as a
dispersion or suspension.
The hydrotransport process is known as the "hot water process" for extraction.
Slurry temperatures in commercial processes generally range from about 450 C
to
600 C. The contact time of the solution of zeolite with the ore depends on the
length of the pipeline and pumping rate, and can be 10-30 minutes long.
Contact times that are either longer or shorter than this are also
contemplated.
In figure 1, the zeolite/hot water solution is mechanically mixed with the ore
to
break up any lumps of ore and the slurry flows down the pipeline. At the end
of
the hydrotransport pipeline, the bitumen (now called froth) is separated from
the
slurry by flotation in the primary separation unit. Then the froth is
deaerated
and flows to a further purification step called froth treatment. Exiting the
froth
treatment system is a deaerated bitumen stream, ready for upgrading by
catalytic
processes.
The zeolite that is present in the hydrotransport slurry will sequester any
hardness cations such as magnesium and, of particular interest, calcium, that
are
present in the slurrying water and any additional hardness ions that may be
released from the ore during hydrotransport. The zeolite is therefore
available
immediately to sequester calcium in the ore as it is slurried and is also
available
to sequester any residual calcium released from the ore during hydrotransport.
In this fashion, the zeolite particles are "sacrificial" in that they are
inactivated
over time as they bind to the calcium present in the slurry to prevent
formation
of calcium naphthenate. Similarly, the zeolite particles may bind to other
multivalent cations to prevent formation of other multivalent-cation
naphthenates.
The resulting bitumen can then be more effectively treated with solid zeolite
catalysts, downstream without "poisoning" such solid catalysts.
9
CA 02944898 2016-10-07
The invention is further described and illustrated by the following examples.
In
the examples, laboratory extractions of bitumen from oil sands ore are
performed using a Bitumen Extraction Unit (BEU), the conventional laboratory
procedure for determining bitumen extractability, and defining ore quality.
Zeolite is produced by the procedure in '933. Caustic and zeolite are added,
either singly or in combination, to the ore slurring water used in the BEU,
simulating chemical addition at the head of the 'hydrotransport' pipeline. An
illustration of a BEU, and its stepwise procedure, are given in Figure 2.
Representative processes for synthetically producing zeolite particulates are
also
described herein.
EXAMPLES
The invention is further described and illustrated by the following detailed
examples, which are not intended to limit the scope of the invention.
Example 1: Reagent Preparation 1 ("instant zeolite")
A first zeolite particle mixture was prepared for evaluation. The process
water
used in the preparation of the zeolite dispersion had a calcium ion
concentration
of about 40 ppm, a magnesium ion concentration of about 10 ppm and a pH of
8.0
To 313 ml of water in a blender was added 6.7 ml of PQ MTM brand sodium
silicate. The mixture was sheared at high speed in the laboratory waring
blender
for five seconds. Separately, 10 ml of Kemira SAX 220 TM sodium aluminate was
mixed with 310 ml of water. The blender was turned on and the diluted sodium
aluminate was added quickly to the sodium silicate mixture, with high shear
mixing in the blender for 3 to 5 seconds, to react the sodium silicate with
the
sodium aluminate. After 3 to 5 seconds, the entire mixture was added quickly
to
1202 ml of water and thoroughly stirred. As described in Nagan '561, this
procedure produces a type A zeolite with the mixing of the two dilute reagents
in
the blender.
CA 02944898 2016-10-07
Dilution with the 1202 ml of water terminates the zeolite-forming reaction and
produces a 0.5 wt% actives zeolite working solution. The resulting "instant"
reaction product is a solution (ie., below opalescence or visible sol size).
The
zeolite particles generated by this virtually instant quenching to 0.5% are
thus
sub-nanometer in size.
Example 2: Reagent preparation 2 (three minute zeolite):
A second working 0.5 wt% actives sol was produced with the procedure of
example 1. The reaction mixture was hand stirred, after the 3 to 5 second high
shear mix, for an additional three minutes before addition to the terminating
water.
The resulting "three minute" product is a visibly opalescent sol and it is
commonly accepted to be four nanometers or larger, as described in Nagan '561.
Example 3: testing
Zeolite particles prepared according to example 2 were tested by contacting a
bitumen ore slurry with an aqueous solution of the zeolite particles of
example 2
that were added to the ore-slurring water.
The BEU Bitumen extraction procedure was followed as per Syncrude Analytical
Methods, 1979. The volume of pre-measured process water was adjusted to
compensate for addition of 0.5% solution of nano particle Zeolite. The
extracted
bitumen is tested using the Syncrude procedure for determining the calcium
naphthenate cntent in the bitumen.
Processing and separation of bitumen ore is performed according to the
following procedure:
1. Remove homogenized oil sand from freezer and allow thawing and
reaching room temperature.
2. Set heating bath to 55 deg C. Heat the process water to 60 deg C
3. Weigh 500 gm of oil sand to nearest 0.1 g and add to Batch Extracction
Unit (BEU)
11
CA 02944898 2016-10-07
4. Add zeolite particles as per dosage to the pre measured process and add
to
the BEU. For 1600 ppm Zeolite dosage: to 500 gms of ore, a mixture of 0.5%
Zeolite (80 mls) and water (155 mls) were added
5. Turn motor on to 300 rpm, raising and lowering motor and impeller
assembly to break any lumps
6. Turn on air to 240 ml/min
7. Start timer for 10 mins
8. When complete turn off the air and flood mixture with rest of the heated
water (735 mls of water were added)
9. Mix for another 10 mins at 300 rpm with no air.
10. When complete skim off primary froth into a pre-weighed bottle using
a
flat edged spatula, cleaning the spatula and BEU surface with a pre-weighed
tissue which is placed in the froth bottle and weighed. Analyze froth for Dean
Stark Analysis
11. Mix the remaining material in BEU for 5 minutes at 600 rpm with air
addition of 240 ml/min to generate the secondary froth.
12. When complete skim off the secondary froth in the same manner as the
primary froth and analyze for Dean Stark Analysis
13. Empty the BEU.
14. Wash the impellor and pot with toluene/IPA mix, collecting residuals in
jar. Wipe with a pre weighed tissue and place in ajar. Toluene wash is
analyzed
for Bitumen which is added to primary froth weight.
Calculations:
Recoveries are found as follows:
.. Primary Recovery % = Wt. Bitumen in Primary Froth (g) x 100
Wt. Total Bitumen (g)
12
CA 02944898 2016-10-07
Secondary Recovery % = Wt. Bitumen in Secondary Froth (g) x 100
Wt. Total Bitumen (g) 100
Total Recovery % = Primary Recovery + Secondary Recovery
The results are shown in Table 1.
Table 1: Bitumen Extraction Results
Hot NaOH 500 500 ppm 750 750 ppm 1600 2400
Water Only ppm Zeolite ppm Zeolite ppm
PPril
Only Zeolite plus Zeolite Plus Zeolite Zeolite
NaOH NaOH
pH 7,8 8,5 9.1 9.9 9.4 10.1 8.9 9.4
eh Primary 44.76 47.32 50.2 49.2 44.6 47.1
55.72 53.9
recovery
%Secondary 22.56 25.54 31,2 31.9 38.3 36.3
26.21 25.4
Recovery
% Total recovery 67.32 72.86 81.4 81,1 82.9 83,4 81.93
79.3
Ca Napthenate 19 22 7 7 7 7 2 7
(PM)
Ore quality: low grade
Ore slurry extraction temperature: 55 deg C
Zeolite particle size: - 4nm
Zeolite dosage: ppm actives (based on weight of
ore)
Nano particle zeolite is shown to be able to reduce the formation of calcium
naphthenate while at the same time raising pH that improves bitumen extraction
Various dosages of zeolite are shown in the top line of Table 1. The notes
under
the table reference the zeolite actives dosages that are being based on weight
of
ore.
13
CA 02 944898 2 01 6-10-07
. .
Example 4
A further test was performed using the zeolite particles prepared in
accordance
with examples 1 and 2. The bitumen extraction, processing and separation steps
were identical to the steps of example 3.
Table 2 tabulates the results of example 4. Table 2 incorporates data from
example 3 (table 1) and adds additional data
Table 2:
Hot NaOH 500 500 500 500 750 -- 750 ppm
Water Only ppm ppm ppm ppm ppm <nm Z
Only 40m 4nm <nm <nm 40m 750 ppm
plus plus
NaOH NaOH 40m Z
Plus
NaOH
pH 7.8 8.5 9.1 9.9 9.0 9.9 9.4 10.1 9.3
% Primary
44.76 47.32 50.2 49.2 49.8 50.6 47.1 44.2
recovery
Secondary 22.56 25.54 31.2 31.9 30.6 29.5 38.3 36.3 37.7
Recovery
%Total
67.32 72.86 81.4 81.1 80.4 80.1 82.9 83.4 81.9
recovery
Ca
19 22 7 7 11 11 7 7 9
Naphthen
ate (ppm)
1600 ppm 1600 ppm 2400 ppm 2400 ppm
40m Z <rim Z 4nm Z <0m Z
pH 8.9 9.1 9.4 9.5
% Primary
recovery 55.72 54.1 53.9 52.9
% "
Secondary 26.21 25.83 25.4 25.2
Recovery
%Total
81.93 79.93 79.3 78.1
recovery
Ca
Naphthen 2 5 7 9
ate (ppm)
14
CA 02944898 2016-10-07
=
Ore quality: low grade
Ore slurry extraction temperature: 55 deg C
Zeolite particle size: 4ijm Z = 4tim
<ijrn Z = Sub-Dm
Zeolite dosage: ppm actives (based on weight of ore)
The foregoing description and test results are given for clearness of
understanding only, and no unnecessary limitations should be understood
therefrom, as modifications within the scope of the invention may become
apparent to those skilled in the art.