Note: Descriptions are shown in the official language in which they were submitted.
CA 02829512 2013-09-09
Vacuum System and Endoscopy Arrangement for Endoscopic Vacuum Therapy
The invention herein relates to a vacuum system and an endoscopy arrangement
for endoscopic
vacuum therapy, in particular for endoscopic intracorporeal, intraluminal or
intracavitary vacuum
therapy.
State of the Art
Endoscopic examinations of the upper and lower gastrointestinal tract
(esophagogastroduodenoscopy/
recto-, sigmoido-, ileocoloscopy, small bowel endoscopy) are diagnostic and
therapeutic routine
examinations.
The examination of the middle intestinal tract, especially the small
intestine, is difficult because it is very
long and extremely mobile. On the one hand, extra long endoscopes are used in
the so-called push
enteroscopy, on the other hand, the so-called single or double-balloon
enteroscopy is used. For better
advancement of the endoscope, the latter uses balloon systems on the endoscope
and/or on the
overtube; they are inflated during the examination and can press against the
intestinal wall from inside.
As a result, the endoscope or the overtube can become wedged against the
intestinal wall, thereby
allowing a deeper examination of the intestine. Another possibility of
examining the intestine is a
photographic record via a swallowable video capsule.
Conventional vacuum sponge therapy (low pressure wound therapy) is used for
the treatment of
external wounds. An open-cell polyurethane sponge or other fluid collection
medium is placed into the
wound, sealed by means of a film, and then subjected to a vacuum. Wound
cleansing and wound
healing can take place under this arrangement.
Explanation of the Invention
According to the invention, a vacuum system for endoscopic intracavitary,
intraluminal or intracorporeal
vacuum therapy is proposed for the aspiration of body fluids, wound secretions
or gases from a hollow
space, such as a body cavity, a hollow organ, a tissue abscess or an
intestinal lumen, especially while
establishing a temporary endoscopic closure of an intestinal lumen. The vacuum
system comprises:
¨ A vacuum pump having a control input for receiving a control signal for
control of its suction capacity
and, on the negative pressure side, a connection for a vacuum drainage
arrangement, and,
¨ connected or connectable to the control input of the vacuum pump, a pressure
regulating unit
¨ having a test signal input for receiving at least one pressure test signal
which forms a
measurement for a pressure or negative pressure that prevails at the hollow
space to be
treated,
1
CA 02829512 2013-09-09
¨ and being designed,
upon specification
a) of a negative pressure value at the hollow space to be treated, which is
selectable
from a predefined negative pressure value interval, and
b) of an evacuation period, the value of which is selectable between 0.5 and 5
seconds,
i) figuring in a predetermined dead volume of the vacuum drainage
arrangement that is connectable to the vacuum pump, to determine a first
suction
capacity of the vacuum pump, required for generating the specified negative
pressure at
the hollow space to be treated within the specified evacuation period, and to
transmit a
corresponding first control signal to the control input of the vacuum pump,
ii) upon generating the specified negative pressure at the hollow space to be
treated, to monitor the pressure test signal and to determine, as a function
of the
current pressure test signal, a second suction capacity of the vacuum pump,
required for
maintaining the specified negative pressure, and to transmit a corresponding
second
control signal to the control input of the vacuum pump; and
iii) upon generating the specified negative pressure at the hollow space to be
treated, if a deviation of the measured pressure or negative pressure from the
specified
negative pressure exists that exceeds a predefined threshold of the measured
pressure
or negative pressure, to determine a third suction capacity that is required
for
generating the specified negative pressure within the specified evacuation
period and to
transmit an appropriate third control signal to the control input of the
vacuum pump.
¨ the vacuum pump being designed, as a function of the control signal
currently being applied
to its control input, to generate a suction capacity determined by the control
signal.
Hereinafter, findings on which the invention is based, will first be explained
in greater detail.
Subsequently, exemplary embodiments will be presented.
The invention is based on the finding that the experience of vacuum therapy in
external wounds is not
applicable to endoscopic vacuum therapy. Based on this fact, it recognizes a
rapid build-up of the
vacuum with a short evacuation period as an essential technical prerequisite
that can determine the
success of an endoscopic vacuum treatment.
The requirements of a vacuum pump unit for endoscopic vacuum therapy can be
specified as follows
according to the invention: The negative pressure applied to a fluid
collection element must be rapidly
sufficiently high, so that the fluid collection element can be aspirated and
adhere firmly to the
surrounding tissue. The vacuum must not be too high, so that, via an open-pore
structure of a fluid
collection member to be connected, it can achieve a drainage effect on the
surrounding tissue. The
suction effect must not cause any injury to the aspirated tissue. In such
cases, the adequate drainage
effect at the wound is absent.
2
CA 02829512 2013-09-09
The suction capacity of the vacuum pump is, therefore, designed according to
the invention herein and
adjustable by means of the pressure regulating unit in such a way that a
vacuum defined under these
marginal conditions, can be built up within a very short period of time or at
a rapid speed and can be
maintained constant. The therapy would be ineffective if the parameters
concerning the vacuum build-
up were not to correspond to the specific requirements. Only with a rapid
vacuum build-up and its
maintenance and, rapid restoration of the vacuum in case of need, for example
with a typical treatment
indication, namely, the treatment of esophageal injuries, a closure of the
perforation defect and
effective wound drainage will be implemented at the same time. The permanently
secured closure and
the drainage against the physiological intrathoracic negative pressure in the
direction of the esophageal
lumen stops contamination by saliva or secretions toward the chest cavity and
thus acts as a barrier to
infection. In the case of any longer vacuum build-up and even with short
interruptions of the specified
negative pressure, a dislocation of a fluid collection element may occur. Any
interrupted or ineffective
suction at the wound bed leads to a standstill in therapy or deterioration of
the wound situation. With
intraluminal treatment in the esophagus in case of an esophageal perforation,
the loss of the negative
pressure can allow swallowed tough saliva secretions to get between the
esophageal wall and the fluid
collection element and lead to clogging of the pores and hence to
discontinuation of the therapy. In case
of other applications in the small or large intestine, insufficient pressure
parameters can cause clogging
of the pores by small or large intestine feces.
In endoscopic vacuum therapy, according to thefindings of the invention
herein, a permanent negative
pressure is generated at the wound, avoiding a drop in the negative pressure
subject to the therapy. In
case of a sometimes inevitable drop of the negative pressure, the vacuum is
very rapidly restored by the
vacuum system according to the invention. For example, when placing a sponge
drainage into the
esophagus, as a result of the physiological swallowing action, a drop in the
vacuum can occur both
because of the swallowing of saliva, food, air and gas and because of
intestinal peristalsis. The same
applies to the application of vacuum therapy on the small intestine, large
intestine or stomach or in the
entire intestinal system.
In contrast to the vacuum treatment of external wounds, in endoscopic vacuum
therapy there is no
possibility of visual and palpatory monitoring to determine whether the
negative pressure is being
applied to the fluid collection element and the internal wound because the
fluid collection element
beneath the surface of the body can no longer be seen after placement. The
therapy come to a standstill
or the therapy is ineffective if the exertion of suction on the wound is built
up too slowly or is
interrupted. An interruption of suction may also occur as a result of
clogging, kinking or collapse of the
fluid communication element or fluid collection element. Clogging of the open-
pore structure as a result
of the tissue aspiration may also occur in case of too high a vacuum, and with
an elastic fluid collection
element, a complete collapse of the fluid communication element may occur upon
elimination of the
suction effect on the tissue. It is only by the characteristics of the vacuum
system according to the
invention, specified in the invention, that an effective internal vacuum
therapy is made possible.
The endoscopic vacuum therapy made possible by the invention involves a
negative pressure therapy
for internal wounds carried out using flexible endoscopes subject to
endoscopic vision and using
endoscopic techniques, vacuum drainage devices being intracorporeally
introduced into hollow spaces
in cavities (intracavitary), intestinal lumens (intraluminal) via natural or
artificial body orifices. Hence,
the vacuum system according to the invention enables endoscopic vacuum therapy
of internal wounds,
3
CA 02829512 2013-09-09
some of which, if untreated, are associated with a high mortality rate or
often require complex surgical
treatment.
In contrast to the vacuum treatment of external wounds, in the endoscopic
intraluminal, intracavitary
and intracorporeal vacuum therapy using the vacuum system of the invention
herein, it is only by
mutual attachment of wound and soft part tissue around the fluid collection
element, which, in surgical
use, conveying fluids, is connected to the vacuum pump of the vacuum system
according to the
invention, after application of the negative pressure, that internal wound
sealing is achieved. Only as a
result of this tissue sealing will the hermetically sealed space be created
that allows the lasting
generation and maintenance of a vacuum. The hollow space, in which the fluid
collection element is
located, is evacuated and, as a result of the vacuum, collapses above the
fluid collection element. If the
fluid collection element is elastically designed, it, too, collapses as a
result of the vacuum. Using the
vacuum pump of the vacuum system according to the invention, a permanent
suction effect is
generated on the wound, which is closed thereby. The fixing of the fluid
collection element at the
placement site is not achieved until the adjacent tissue and/or the intestinal
mucosa becomes attached
by suction.
In contrast to the vacuum therapy on external wounds, in which sealing is
performed using an occlusive
film dressing, sealing in endoscopic vacuum therapy, which can only take place
because of the vacuum
as a result of the abutting tissues, is less stable. Sealing and, therefore,
the fixing of the fluid collection
medium is exclusively caused by the fact that, as a result of the vacuum, the
fluid collection element
attaches itself to the tissue by suction similar to a suction cup and the
vacuum is maintained
permanently and constantly. The invention is, therefore, based on the finding
that, for successful
implementation of endoscopic vacuum therapy, a vacuum system must meet the
requirements specified
by, set on and monitored by the pressure regulating unit.
For build-up of a vacuum for carrying out any vacuum endoscopy, in the vacuum
system according to
the invention, a vacuum pump is provided, the suction capacity of which is
controllable and which is
designed to generate, within a short defined evacuating period of between 0.5
and 5 seconds, a
specified negative pressure at the application site of the fluid collection
element and then to maintain it
at a constant value.
The invention is further based on the finding that an additional parameter,
namely the volume of the
wound cavity to be evacuated or the intestinal lumen, is negligible.
Conversely, it is noteworthy that
with constant volumes to be evacuated (fluid communication element, fluid
collection element,
secretion container), the rate of the suction build-up in the presently
relevant range of values of the
negative pressure, with the volume of the secretion container being known, can
practically be controlled
via the suction capacity of the vacuum pump alone (liters/minute). Since the
secretion collection
container can fill up with secretions, the dead volume is then reduced. This
can also be measured, and
the suction capacity can be automatically adjusted to the filling state of the
container.
Hereinafter, exemplary embodiments of the vacuum system according to the
invention are described.
In the specification herein, negative pressure values are provided relative to
ambient pressure. In the
literature, these negative pressure values are often also provided with the
negative sign. It will be
omitted herein, and only the amount of negative pressure will be indicated. As
is customary in
professional circles, the negative pressure values will be given in units of
mm Hg, and for the purpose of
4
CA 02829512 2013-09-09
conversion to SI units, a ratio of 1 mm Hg = 133.322368421 Pa can be used. The
term vacuum is used in
the specification herein as a synonym for the term negative pressure.
The inventor has found that, in practice, negative pressures of an amount of
less than 60 mm Hg and an
amount greater than 500 mm Hg are not be required and that insofar, the
performance of the vacuum
pump can be limited in favor of a design of limited performance but instead
lighter and preferably
portable by the patient.
As a vacuum pump, in various exemplary embodiments, a displacement pump, such
as a rotary piston
pump, a rotary vane pump, a trochoid pump, a scroll pump, a piston pump, a
helical pump, a rotary
piston pump, a roller pump or a membrane pump is provided.
The vacuum pump to be provided in the vacuum system according to the invention
shall, within the
context of the specification herein, be understood to include combinations of
at least two pumps or
multi-stage pump systems. In one embodiment, the vacuum pump is for example
equipped with two
pumping stages. In this arrangement, the vacuum pump is preferably equipped
with a pump
combination. Preferably, the vacuum is generated via a prevacuum using a
booster pump,
A parameter of the vacuum system according to the invention that is important
for successful treatment
is the period of time required for evacuation to the required negative
pressure of the volume involved in
each case in the sections of the hollow spaces to be treated. In preferred
embodiments, the maximum
suction capacity of the vacuum pump is designed in such a way that, taking
into consideration the dead
volumes that occur in practice, the pressure value of the vacuum specified
according to the invention is
reached within a short period of about half a second.
In other embodiments, the maximum possible evacuation period lasts up to a few
seconds, in particular
up to a maximum of 2 seconds, in order to reach a defined continuous vacuum.
Accordingly, in the
mentioned embodiments of the vacuum system, the pressure regulating unit is
designed to control the
vacuum pump in operation for achieving the vacuum within a range of values of
the evacuation period
that comprises the stated minimum and maximum values of the evacuation period.
The negative
pressure is maintained constant after the evacuation period.
Advantageous embodiments of the vacuum system additionally have, connected to
the pressure
regulating unit, a user input unit which is designed to accept a user input of
the evacuation period
and/or a negative pressure value and to transmit it to the pressure regulating
unit. The pressure
regulating unit is designed to determine the control signal concerned,
figuring in the current user input,
and transmit it to the control input of the vacuum pump.
In a variant, for exceptional situations, additionally, the operation of the
vacuum system with an
evacuation period of more than 5 seconds is possible via an appropriate user
input on the user input
unit (or on a hand piece or foot pedal, connected to the user input unit, to
be operated by the
physician.) This can also be useful if, using a single pump system, the
present focused vacuum
endoscopy as well as a vacuum treatment of external wounds is to be feasible.
A certain temporary increase of the vacuum over the value intended for therapy
may be indicated
initially, hence at the beginning of therapy, for a short period of time, in
order to assure a secure
CA 02829512 2013-09-09
attachment of a drainage device at the therapy location. Even after placement,
initially a higher negative
pressure is temporarily advantageous so that the fluid collection medium can
be suctioned into place in
such a way that it cannot accidentally be dislodged by an endoscope introduced
into the body during
this initial phase. But if so, in comparison to the total duration of therapy,
this involves a relatively short
initial period of time, for instance, 15 minutes, while the duration of
therapy can typically extend over
several days.
After successful placement, the patient will typically carry the vacuum system
with him. Preferably, the
user input will have a lockable mode switch that allows adjustment by the user
of either a therapy mode
or an endoscopy mode, the pressure regulating unit being designed to output
only the second or third
control signal but not the first control signal in the therapy mode and the
predefined negative pressure
value interval in the therapy mode extending over negative pressure values
with respect to a
surrounding pressure between a minimum negative pressure of 60 mm Hg and a
maximum negative
pressure of 250 mm Hg. The locking of the mode switch is preferably only
possible using a key, wherein
key can also mean a code.
According to a finding of the inventor that led to an enhancement of the
invention, the success of the
therapy is further improved if the evacuation period and the negative pressure
are adaptable according
to an examination or therapy to be carried out in each case. In the treatment
of esophageal injuries, for
instance, the pathophysiological intrathoracic negative pressure and the
pressure fluctuations caused by
respiratory motion are directed against the suction effect of the vacuum pump.
This physiological
negative pressure must be cancelled out or counteracted by a very short vacuum
pump evacuation
period in the direction of the suction of the pump. In contrast to the therapy
on the esophagus, in case
of a negative pressure treatment following anastomotic insufficiencies at the
rectum, with an Anus
praeter upstream and only very slight secretion, a longer evacuation period
within the limits of the
invention of up to 5 seconds may also be therapeutically successful.
After the vacuum has been built up to the defined pressure, this pressure must
be maintained constant.
A drop in the negative pressure is immediately recordable by the vacuum system
according to the
invention using the sensor and removable again within the evacuation period,
advantageously, as a
result of the usually remaining residual vacuum, a period of time even much
shorter than the evacuation
period being needed for restoring the nominal value of the vacuum. In this
way, a constant negative
pressure can be assured at the therapy location. Preferably, the pressure
regulating unit and the vacuum
pump are, therefore, designed for being able to build up the defined negative
pressure as a function of
incoming test signals at a frequency of at least 30 vacuum buildups/minute.
This proves to be beneficial
for carrying out an endoscopic vacuum sponge therapy on the upper
gastrointestinal tract. In further
embodiments, using the vacuum system, up to 60, more preferably 120 vacuum
buildups/minute can be
carried out.
In a preferred embodiment moreover, the evacuation period is adjustable by
user input via the pressure
regulating unit. For this purpose, the vacuum system additionally has,
connected to the pressure
regulating unit, a user input unit which is designed to accept a user input of
the evacuation period and
to transmit it to the pressure regulating unit. The pressure regulating unit
is adapted to control the
vacuum pump as a function of the user input, in order to generate the negative
pressure in the specified
evacuation period.
6
CA 02829512 2013-09-09
In an additional embodiment, the pressure regulating unit is designed to
support a selection between
the following predefined therapy settings via the user input unit by
appropriate predefined control
parameters:
a) a maximum evacuation period of 2 seconds;
b) an evacuation period between 0.5 and 5 seconds more closely definable by
further user input;
c) an adjustable evacuation period of 2 -5 seconds.
For example, for the treatment of an esophageal leakage, a negative pressure
between 80 and 150 mm
Hg (10665 to 20000 Pa) and selecting a maximum evacuation period of 2 seconds
is advantageous.
In many applications, the value of the negative pressure to be selected also
depends on a contact
surface of a fluid collection medium with the surrounding tissue. With a large
contact area, compared to
a small contact area, for fixing the fluid collection medium a lesser negative
pressure may be required.
The user input unit is, therefore, preferably additionally designed to receive
an additional input of an
identification of a type of fluid collection element. In this embodiment, the
pressure regulating unit is
designed to determine, based on prestored therapy data, values assigned to the
input type of the fluid
collection element of the vacuum and/or the evacuation period, and to control
the vacuum pump during
operation in accordance with these determined values.
In clinical applications, a continuous lasting negative pressure has
essentially proven to be of value. But
a variant provides for the pressure regulating unit to be designed to control
the vacuum pump so as to
apply the negative pressure fluctuating between at least two negative pressure
values, for example
between about 100 mm Hg and about 150 mm Hg. By applying a fluctuating
negative pressure, the
granulation stimulation of the wound can be increased. However, at no time
must a suction interruption
lasting longer than a few seconds take place.
The pressure regulating unit is designed to monitor negative pressure values
during a negative pressure
application and an examination carried out using negative pressure. Sensors
are connectable by either
electrical, i.e. wired, or wireless communication to the pressure regulating
unit of the vacuum system,
so that preset negative pressure values of the vacuum pump to be generated can
be monitored and
adjusted by the pressure regulating unit. In this way, as a result of the
pressure detection, monitoring
and control of the pump suction can be performed directly on a fluid
collection element by sensors. This
prevents a therapy standstill from occurring, for example in case of clogging
of the fluid collection
medium or the fluid communication element. This is particularly important in
the treatment of
esophageal injuries, because otherwise an inflammation of the chest cavity
occurs, which entails a
tedious treatment and often leads to death.
By evaluating the test signals arriving from the pressure sensors, comparing
them to a nominal value in
each case, the pressure regulating unit preferably also captures the
evacuation period actually required.
The vacuum system is preferably provided with a vacuum drainage arrangement
which is connected
upstream of the vacuum pump on the negative pressure side. With the same
suction capacity of the
7
CA 02829512 2013-09-09
vacuum pump, rapid suction build-up is possible using a smaller secretion
collection container rather
than a large collection container. The pressure regulating unit is, therefore,
preferably designed to
accept a user input of a collection container volume via the user input unit
and to adjust the pump
capacity additionally dependent on the entered volume. In doing so, the
pressure regulating unit
controls the pump capacity not only, as explained above, in accordance with
the evacuation period
desired by the user, but also additionally takes into account for this purpose
the volume of the secretion
collection container.
The pressure regulating unit is designed to figure in a secretion collection
container volume as part of
the dead volume. Depending on the application, different secretion collection
container volumes may be
required so that the pressure regulating unit must be able to use
appropriately different dead volume
values. They may, for instance, be saved in a memory of the pressure
regulating unit and selected by
user input. To prevent input errors, alternatively coding, affixed to the
secretion collection container per
se and readable by the pressure regulating unit, may be captured, from which
the applicable dead
volume value can be derived. With a small collection container volume, the
suction capacity of the pump
is set lower, with a larger volume, the suction capacity is set accordingly
higher.
The secretion collection container is designed to accept and/or discharge
secretions and gas that occurs
during operation and is aspirated by the vacuum pump. Preferably, the pump is
additionally equipped
with a negative pressure-resistant presecretion collection container, which is
connected, in the direction
of suction, upstream from the application site on the patient toward the
vacuum pump and is connected
to the secretion collection container conveying fluid. The pressure regulating
unit is then designed
appropriately to figure in a secretion collection container volume as an
additional part of the dead
volume.
In this variant, collected secretions can be conveyed from the presecretion
collection container to the
secretion collection container. Preferably, the presecretion collection
container and the secretion
collection container are connected to each other across a valve. As an
addition or an alternative to the
valve, the presecretion collection container and the secretion collection
container are connectable to
each other via an interposable filter. Preferably, these collection containers
and their connection to the
vacuum are designed exchangeable.
These embodiments provide for a suction build-up via the dead volume of the
secretion collection
container. If the suction build-up of the vacuum pump takes place via a
secretion collection container,
its dead volume, together with the suction capacity of the pump (L/min),
substantially determines the
rate of suction build-up. The suction capacity of the vacuum pump is,
therefore, preferably designed to
evacuate additionally the dead volume which is formed by the secretion
collection container and the
presecretion collection container within the evacuation period.
The secretion collection container is connectable, via preferably negative
pressure-resistant fluid
communication elements, in particular drainage hoses, to a fluid collection
element so that the negative
pressure at the fluid collection element can be built up via the secretion
collection container. The
pressure regulating unit is designed to figure in, as an additional portion of
the dead volume, an
additional volume, which forms at least one negative pressure-resistant fluid
communication element,
in particular a drainage hose, which is distally connectable to a fluid
collection element and proximally
to the secretion collection container or the presecretion collection
container. With constant evacuation
volumes on fluid communication elements, fluid collection elements and
secretion collection containers,
8
CA 02829512 2013-09-09
in one embodiment, the evacuation period can be adjusted on the pressure
regulating unit via an
adjustment of a suction capacity of the vacuum pump. In this arrangement, the
pressure regulating unit
of the vacuum pump receives, in addition to the user input, as an additional
input for adjustment via the
test signal input, measured values from a negative pressure sensor, which is
located by the hollow space
to be evacuated. Details concerning the embodiment and placement of the sensor
are discussed below.
If a presecretion collection container used, in these embodiments it is
preferably connected to the
vacuum pump in such a way that it can be subjected to a prevacuum. In this
embodiment, the vacuum
pump has two pump stages and is designed to generate the negative pressure at
the
examination/treatment site using a first stage of the two pump stages via a
prevacuum in the
presecretion collection container. It has the comparatively smaller volume of
the two collection
containers, in order to achieve as short an evacuation period as possible. The
presecretion collection
container typically has a volume of 50 mL to 300 mL. The secretion collection
container, on the other
hand, typically has a volume of 100 mL to 1000 mL. But smaller or larger
volumes can also be selected,
subject to adjustment of the required suction capacity of the vacuum pump.
An additional preferred embodiment provides for a pressure regulating unit,
which not only adjusts the
capacity of the pump in accordance with the volume of the secretion collection
container, but also takes
into account a volume of a fluid collection element to be evacuated. Here
again, as described above, an
additional user input via the user input unit is provided, which is forwarde
to the pressure regulating
unit, which, in turn, appropriately controls the pump capacity for achieving
the evacuation period in
each case. In doing so, an evacuation of the secretion collection containers
is simultaneously carried out
within the evacuation period.
Preferably, the pump capacity is designed in such a way that, within the
evacuation period, the dead
space volume of the secretion collection container and the fluid collection
element is evacuated.
Experience hitherto shows that a controllable pump capacity in the range of 1
L/min to 20 L/min is
required.
In one embodiment of the vacuum system, the pressure regulating unit is
equipped with a monitoring
unit, which automatically monitors any excessive rise and/or reduction of the
negative pressure, of the
duration of the evacuation period as well as a duration of a negative pressure
system and adjusts the
pump capacity if specified limit values are exceeded. Thus, during operation,
in case of any drop of the
negative pressure, for example as a result of an insufflation of examination
gas, the vacuum can be
rapidly restored and, as a result, continuously maintained.
In addition to the control signal input, further switching and control
elements, across which an
operation of the vacuum pump can be carried out, are preferably provided on
the vacuum pump. In
particular, the pressure regulating unit and the user input unit can be
integrated as a structural unit with
the vacuum pump.
For capturing and monitoring the defined negative pressure and the evacuation
period, at least one
negative pressure sensor on the vacuum pump and/or at least one connection for
an external negative
pressure sensor is provided. The negative pressure sensor is directly or
indirectly connected to a fluid
collection medium and/or fluid communication element that is connected to the
vacuum pump and is
9
CA 02829512 2013-09-09
designed to forward its test results as test signals to the pressure
regulating unit of the vacuum pump.
Preferably, the fluid communication elements are drainage hoses.
Using the vacuum pump, a vacuum can be built up on a single fluid collection
element or a plurality
thereof. For the case of a plurality of fluid collection elements, the vacuum
pump is preferably designed
for accomplishing any vacuum generation completely independent from each
other. For this purpose,
not only a plurality of appropriate connections and drainage units are
provided. In addition, the pump
capacity of the vacuum pump is adapted to the higher demands of simultaneous
negative pressure
generation on various fluid collection elements. The pressure regulating unit
is designed to output
control signals to individually controllable throttle elements which are
arranged in each corresponding
branch, in order to effect the individually adapted build-up of a vacuum in
each case.
In particular, the vacuum pump is designed to generate a vacuum in endoscopic
intracavitary and
intraluminal vacuum therapy. It is, however, also usable in vacuum sponge
therapy on external wounds.
It is moreover usable in vacuum endoscopy.
The vacuum system is preferably designed as a portable unit, so that a patient
can move as freely as
possible and be mobile. In the portable version, the electrical power supply
of the pump is assured for
example by a battery or battery pack.
It should be noted that in an alternative embodiment, the vacuum pump in
treatment rooms may exist
in the form of an integrated, centrally controlled vacuum wall suction device,
which must be
appropriately adjusted in its pumping capacity, in order to supply at least
the vacuum required in
accordance with the invention within the evacuation period necessary according
to the invention.
In this way, with an appropriate infrastructure, in a treatment room, the
generation of the necessary
vacuum can take place even without a separate vacuum pump, hence with the
appropriately designed
wall suction device replacing the vacuum pump. The pressure regulating unit of
the vacuum system
according to the invention must be adapted in such an infrastructure, in order
to be able to control
vacuum pressure control elements, such as throttle elements depending on the
given (usually not
controllable) pumping capacity of the wall suction device as a function of
time, so that the required
negative pressures between fluid collection element and wall suction device
are reached within the
specified period of time. Via connection, filter, switching and valve element,
the forwarding of a vacuum
to the fluid communication elements can be made possible and the operation via
the handle of the
endoscope can take place.
Advantageously, the user input unit of the vacuum system comprises an
arrangement for manual
control of the vacuum pump, by means of which a start signal for starting and
a control signal for
reduction of the vacuum on the fluid collection element for forwarding to the
pressure regulating unit
can be generated and output. Preferably, the user input unit is connected to
one switching device or a
plurality thereof on the handle of the endoscope; alternatively, operation of
the pump via foot/hand
switch or directly at the pump is also possible.
In order to be able to perform the examination, in particular a vacuum
endoscopy, comfortably, it must
be possible to build up and release the negative pressure at short intervals
during the course of the
examination. For this purpose, in preferred embodiments, a switching unit on
the endoscope or a foot
switch is provided.
CA 02829512 2013-09-09
In one embodiment, the vacuum system has a plurality of negative pressure-side
connections for one
drainage hose or a plurality thereof. The pressure regulating unit in this
embodiment is designed to
control the vacuum pump upon an appropriate user input via the user input
unit, optionally aspirating or
flushing either unilaterally only one of the connections or alternating two of
the connections or
simultaneously two connections. In this way, the vacuum on a plurality of
fluid collection elements can
be controlled simultaneously and independent from each other, which will be
explained in more detail
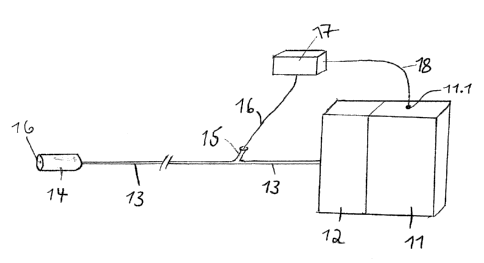
below within the framework of the description of the figures.
The vacuum system according to the invention in preferred embodiments forms a
technical component
of an endoscope arrangement according to the invention having
- such a vacuum system according to the invention or one of its exemplary
embodiments
described within the framework of the application herein,
- an overtube unit, which, on the negative pressure side, is connected to
the vacuum pump of the
vacuum system by at least one fluid communication element and has a fluid
collection element,
- an endoscope which is inserted or insertable into the overtube unit and
is displaceable relative
to overtube unit in a direction going from proximal to distal or vice-versa
and
- a negative pressure sensor which is connected to the pressure regulating
unit of the vacuum
system.
Preferably, the endoscope is also connected to the vacuum pump of the vacuum
system via a fluid
communication element on the negative pressure side and has an additional
fluid collection element.
This exemplary embodiment according to the invention in the form of an
endoscopy arrangement is
based on the finding that in balloon enteroscopy, known in the state of the
art, adequate fixing of an
endoscope or overtube by clamping the balloon to the intestinal wall is
frequently not possible and, as a
result, deeper examinations do not succeed. The balloon can easily slip; in
particular, sufficient fixation
in the case of wide intestinal lumens (stomach/colon) is not possible. If the
balloon is excessively
inflated, there is a risk of intestinal wall injury and even wall rupture.
The endoscopy arrangement utilizes this finding for embodiment of an endoscopy
arrangement for
endoscopic intraluminal sponge vacuum therapy, in order to place one fluid
collection element or a
plurality thereof, for example sponge drainage devices, for example
intraluminally, in the intestinal
lumen and to anchor them at the placement site using a vacuum according to the
parameters specified
according to the invention. The sponge drainage devices in this example, with
the vacuum applied to the
sponge, attach themselves by suction to the intestinal mucosa and are fixed to
the placement site by the
negative pressure.
Using alternating mutual displacement of an overtube relative to an endoscope
introduced into it, it is
possible to push an endoscope forward in the intestine. Endoscope and overtube
need an anchoring
arrangement against the adjacent tissue, such as the intestinal mucosa. In the
embodiment of the
endoscopy arrangement herein, this anchoring is achieved by the attachment by
suction of the sponge
11
CA 02829512 2013-09-09
drainage device to the intestinal mucosa. For this reason, the treatment or
examination method based
thereon is also referred to as a vacuum endoscopy.
Endoscopic vacuum therapy is used in the treatment of internal wounds. Their
effectiveness was first
demonstrated in suture leaks at the rectum, then also in the case of
intestinal leakages at other
locations, such as esophagus, stomach, small and large intestines. In the case
of internal wounds,
cavities, abscesses, empyemas, fistulas situated below the skin surface and
which are or are made
endoscopically accessible via an opening to the outside, endoscopic vacuum
therapy can also be used
for wound treatment. In endoscopic vacuum therapy, the natural or artificial
means of access to hollow
organs, gastrointestinal tract and body cavities are used endoscopically.
Using the endoscope, sponge
drainage devices are introduced internally, intracorporeally, intraluminally
and intracavitary. In the
intraluminal therapy variant, the sponge body is placed in the intestinal
lumen at the defect level. In the
intracavitary variant, the sponge body is introduced through the defect into
an (extraluminal) wound
cavity. Both therapies may also be combined. After the sponge body is
positioned, vacuum suction is
applied to the led out drainage hose. The wound cavity or the intestinal lumen
collapses subject to the
suction together with the elastic sponge body. The sponge surface attaches by
suction to the wound
surface suction cup-like, and, at the same time, it fixes itself at the
placement site by suction. Effective
wound drainage takes place, at the same time the wound defect is closed.
Subject to the lasting
drainage effect and vacuum application to the wound surface, the wound
cleanses itself, granulation
tissue forms and the wound heals as a secondary consequence. An endoscopic
exchange of the sponge
drainage device is performed at multi-day intervals.
A special form of endoscopic vacuum sponge therapy does not aim at complete
closure of a cavity, as
explained above, but at maximum secretion discharge In it, the sponge drainage
device is also placed
into a hollow organ, e.g. the duodenum (postpyloric vacuum duodenal drain) and
subjected to suction.
In doing so, the drainage effect is metered in such a way that complete
intestinal sealing need not be
achieved, but that the fluid collection medium becomes subject to suction to
such an extent that
optimal fluid conveyance is achieved (in the example of a duodenal placement,
of pancreatic and biliary
secretions from the intestinal lumen). It is conceivable to use this type of
application in other hollow
organs or cavities, where maximum secretion drainage is desired.
Using the invention, complete lasting evacuation of the stomach can, for
instance, also be achieved.
From the invention arise numerous innovative therapy possibilities for the
treatment of internal
wounds.
Hereinafter, enhancements of the endoscopy arrangement are described.
The vacuum pump is preferably connected to the sponge drainage unit by one
fluid communication
element or a plurality thereof in the form of drainage hoses and/or in the
form of a channel in the
endoscope, which may also be arranged, at least partially, in or on the sponge
drainage unit. Particularly
preferred, the fluid communication element is fluid-conductive and connected,
via orifices in its wall, to
the fluid collection element. These perforation openings are particularly
advantageously located in a
section between the proximal or distal end of the hose. The perforation
openings are advantageously
located in the middle section of the fluid communication element. In one
embodiment, the perforation
openings are arranged in a plurality of sections between the proximal and the
distal end of the hose. The
perforation openings preferably have a diameter of 1 mm to 10 mm. Above the
perforation openings of
12
CA 02829512 2013-09-09
the hose wall, the fluid collection medium can be attached from the outside by
means of gluing, suture
or another means of attachment.
In enhancements, such fluid communication elements are equipped with dual
lumen or even multiple
channels. Such a fluid communication element is suitable for flushing and
aspirating via various
channels. At least one of the channels is preferably designed in its diameter
in such a way that a wire-
like negative pressure sensor can be temporarily or permanently introduced
into the fluid
communication element.
As a particular advantage, one half of the fluid communication element may
have a small lumen and the
other half a large lumen. This may be particularly advantageous especially
when the drainage device can
be placed in such a way that, for example in the presence of an
esophagocutaneous fistula, one of the
legs of the drainage device discharges percutaneously outward via the
cutaneous fistula and the other
drainage leg inward orally via the esophagus. The of the fluid communication
element leading out can
be closed using clips. Via the fluid communication element, particularly a
flushing treatment can also be
carried out. In particular, in case of placement of the fluid collection
element in the middle section and
both fluid communication legs leading out, one of the legs can be used for
suction, the other one for
flushing.
Advantageously, the various diameters of the fluid communication element are
continuously tapered
and pass from the large lumen to the small lumen diameter without any
gradation. This assures
atraunnatic placement of the drainage device. The perforation openings are
located in particular at the
distal end of the hose. In order to facilitate the introduction of the sponge
system, a wire-like element
can be introduced into the fluid communication element.
Advantageously, the fluid collection elements and the fluid communication
elements are radiopaque.
Preferably, the fluid communication elements have an inside diameter of 1 mm
to 10 mm. Preferably,
the fluid collection element, having approximately cylinder shape, has an
outside diameter of 5 mm to
30 mm.
Larger fluid collection element diameters are, for instance, advantageously
usable, if an intestinal lumen
of a large inside diameter (such as the stomach or colon) are to be closed.
Smaller diameters of the fluid
collection element and the fluid communication element are, for instance,
advantageously usable when
small lumen fistula ducts are to be closed and drained.
The outside diameter of the fluid communication element and the fluid
collection element are, in one
embodiment, adapted to the inside diameter of an inner working channel of the
endoscope, so that
they are displaceable within the inner working channel and their placement can
be undertaken via the
inner working channel of the endoscope. In particular, this achieves placement
of the drainage device
through small orifices subject to visualization. Furthermore, minimizing the
diameter achieves that,
using the endoscopic techniques, the number of regions that are endoscopically
reachable is increased
and, as a result, can be easily supplied with a vacuum drainage unit.
In an alternative variant, the outside diameters of the fluid communication
element and the fluid
collection element are adapted to the inside diameter of an outer working
channel of the endoscope in
13
CA 02829512 2013-09-09
such a way that they are displaceable within the outer working channel and
their placement can be
undertaken via the outer working channel of the endoscope.
In the overtube, in preferred embodiments, one drainage channel or a plurality
thereof are integrated as
fluid communication medium. They are cylindrical. These drainage channels are
negative pressure-
resistant, so that they do not collapse subject to the applied vacuum. They
are connectable to the
vacuum pump by negative pressure-resistant drainage hoses. In particular, the
drainage channels have,
at their distal end in their walls, an opening or a plurality thereof, which
fluid-conductively perforate the
overtube outward in such a way that fluids and gases can be drained by
suction. At the level of the
openings of the drainage elements, the fluid collection element is attachable
or attached, for example
by gluing, string or clamping.
The fluid collection element that is fluid-conductive and connected to the
fluid communication element,
can be placed both endoscopically, laparoscopically, thoracoscopically,
intraluminally in open surgery,
intracavitary, intracorporeally. In an exemplary embodiment, the sponge
drainage unit is attached by
the distal end of the endoscope and/or the distal end of the overtube unit.
For the placement of sponge
drainage devices in deeper-situated regions of the body, such as the colon,
the esophagus or the
duodenum, some having very curvy access routes, a drainage hose is proposed,
to the end of which the
sponge drainage unit in the form of a polyurethane sponge body is sewn.
Preferably, the sponge
drainage unit has a circular or hollow cylindrical, hence tubular, base body.
It consists for example of an
open-pore elastic compressible polyurethane sponge body. Preferred is a pore
size in the polyurethane
foam body from 200 m to 1000 p.m, a pore size of 400 rn to 600 tirn being
particularly preferred. The
sponge can be adapted to the requirements by cutting its length and volume to
size.
In a preferred embodiment, the fluid collection medium is an open-pore film.
Alternatively, a
polyurethane sponge body can be enclosed in such an open-pore film. After
adjusting the length of the
sponge, the film may, for instance, be pulled over the sponge which is
typically achieved by cutting. For
this purpose, the open-pore film is preferably designed as a small baggie and
can be tied closed using a
string. The film may have a structure comprising two film sheets, which are
fluid-conductive and
connected, via pores, over their entire surface.
As mentioned above, the length and thickness of the fluid collection element
can be designed variable.
For example, the fluid collection element in various embodiments is between 2
and 10 cm long, but
other lengths are also possible depending on the application, as indicated
below. In other embodiments,
the fluid collection element has an outside diameter of 1.5 cm to 3.0 cm.
Here, too, adjustments outside
this range of values for certain applications may be expedient. For
intracavitary therapy, for instance,
the fluid collection element is preferably 0.5 cm to 1.5 cm in diameter and 1
to 4 cm long. For
intraluminal therapy, however, the fluid collection element is preferably 1.5
cm to 2.5 cm in diameter
and 4 cm to 10 cm long.
Preferably, the central channel in the fluid collection element has a diameter
of 0.5 cm to 1.0 cm but
other diameters are also possible depending on the application.
The sponge body is graspable using grasping tongs, polyp grabbers or loops and
insertable orthograde
subject to endoscopic control. Placement may, however, be technically
difficult. Visibility is restricted.
The internal wound orifices, through which the sponge body is inserted, for
instance, in intracavitary
therapy, are often small and angled and hard to access. The mobility of the
endoscope is restricted by
14
CA 02829512 2013-09-09
the sponge drainage device. The spaces to be endoscoped are narrow. A blunt-
ended sponge drainage
device easily snags on the internal wound orifice or the intestinal mucosa.
The drainage hose, therefore,
preferably ends distally in a tip.
It is particularly advantageous if the tip of the drainage device is designed
conically as well as, in
particular, soft and atraumatic, in order to avoid injuries of any adjacent
tissue. The pointed-end distal
end of the drainage hose may project beyond the distal end of the fluid
collection element, but it may,
instead, end in the sponge body.
A conically converging configuration of the hose end advantageously continues
in an imposed sponge
body of the sponge drainage unit in such a way that the sponge body
continuously abuts the drainage
device. This facilitates the drainage placement maneuver.
In one embodiment, the projectile-like tapered tip of the drainage hose is
also provided with a central
channel in such a way that, as a result, a guidewire can be introduced.
The tip may advantageously be equipped with a transverse channel, through
which, for instance, a string
can be installed. At the distal end of the drainage hose, at the fluid
collection element or in the fluid
collection element, advantageously a device is attached that can be grasped
using forceps, a hook, a
loop or another insertion instrument. In particular, a string or wire loop may
be attached. In particular, a
grasping bead of metal or plastic may be attached. Alternatively, a metal or
plastic eyelet may be
attached. Or a string may be attached. The string may for example be 1 cm to
250 cm long.
If an additional external access to the internal wound still exists (for
example in the form of a fistula),
the string may be led out via the fistula using an endoscopic technique. If
the tip is lost during the
placement maneuver, the string can be used for recovery. In the presence of an
additional outward
connection, using the insertion instrument or the reinforced string, the pull-
(through) technique can also
be used for placement. The exchange maneuvers can be substantially simplified
by using the pull-
through technique.
The device, which can be grasped using forceps, a hook, a loop or another
insertion instrument is
particularly designed tension-proof in such a way that the drainage device can
be pulled by them
through tissue, intestinal lumens, fistulas. The device must be designed
flexibly and atraunnatically.
It is particularly advantageous if the pointed top-seated attachment is
designed in such a way that, after
application to the end of the drainage hose, the outside of the hose ends
flush with the outside of the
pointed top-seated attachment.
If there is no fistula to the wound outward, by a puncture from the outside,
an additional connection
can be created, across which the string can be taken outside. Furthermore, the
string may be used for
endoscopic, laparoscopic, thoracoscopic or open surgical rendezvous maneuvers.
The intraoperative
placement maneuver can thereby be substantially simplified.
For example, the through-pull technique can be used when placing a sponge
drainage into the
esophagus, when a percutaneous endoscopic gastrostomy to the anterior gastric
wall was installed.
Through this percutaneous stomach access route, a string can be introduced and
taken outside through
CA 02829512 2013-09-09
the mouth using a gastroscope. The string is connected to the tip of the
sponge drainage device and
then, by pulling by the string, pulled to the placement site in the esophagus.
In this way, even bulky and
very long sponge bodies can be atraumatically introduced. Intraluminal
placement becomes much
simpler.
The string is preferably attached to the sponge body or the drainage hose in
such a way that it can be
removed at any time. This is for example possible when the string is passed
through a string loop or an
eyelet in the form of a double string or infinite loop that is attached to the
end of the drainage hose or
sponge body. If the string is to be removed, the infinite loop is severed and
pulled.
Preferably, a longitudinal axis of the sponge drainage unit runs substantially
parallel to the longitudinal
axis of overtube.
Preferably a channel created in the sponge drainage unit (hence, the fluid
collection element)
encompasses the entire circumference of the overtube, a drainage hose
introduced into the sponge
drainage unit having openings there in its wall. However, the fluid collection
element may alternatively
just partially encompass the overtube.
The fluid collection element is advantageously provided with a fluid-
conducting outer coating that
facilitates sliding with respect to the intestinal mucosa in the absence of
any negative pressure.
Advantageously, this outer coating is a fluid-conducting film. In vacuum
endoscopy, the film coating is
advantageously hydrophilic, so that the fluid collection element can slide
more easily on the mucosa. It
is, however, important to assure that the outer coating can conduct the
suction to be applied fluid-
conductively and unabated onto the intestinal mucosa, in particular with the
largest possible surface, so
that the fluid collection element attaches itself by suction and becomes fixed
in place. The suction
effect of endoscopic therapy can only develop on the wound surface in case of
open sponge pores in the
interior region of the sponge drainage unit. If the pores are, for instance,
clogged by mucus, saliva or
tough secretions, no suction effect can develop on the wound. Particularly in
intraluminal treatment of
the esophagus, the sponge body may become partially or completely clogged by
swallowed viscous
saliva. In case of partial clogging, the sponge body does not become attached
by suction to the tissue
across its entire surface but only partially via the open pores. If the pores
become clogged with
secretions, the sponge at these points cannot become attached by suction. It
will be observed that,
between clogged sponge surface and esophageal mucosa, saliva and secretions
may drain even into the
stomach, while during this process the sponge body is simultaneously still
attached by suction to the
mucosa via the pores that remain open.
The airtight delimitation required for vacuum build-up consists, on the one
hand, in contact with the
suction-attached tissue surface, on the other hand, in the surface sealing by
clogging mucus or tough
secretions. Under these conditions, an effective vacuum suction may continue
to exist on the
circumscribed mucosa or wound surface. However, if the pores of the sponge
body are completely
clogged by tough secretions, no suction effect can develop at the wound bed;
the vacuum then exists
only in the fluid-conducting system. Therapy comes to a standstill or there
may even be worsening of
the wound condition.
One embodiment of the endoscopy arrangement, therefore, provides for the
sponge body to have, on
its outer surface, recesses for receiving a sensor, which can be inserted
between intestinal wall and
sponge body during the operation of the endoscopy arrangement. Such an
additional sensor can be
16
CA 02829512 2013-09-09
utilized for enteral feeding, stomach relief or flushing. A vacuum can be
applied to the sponge body
while an additional sensor is simultaneously in place. At the points, at which
the additional sensor
comes to be in place between sponge and intestinal wall, the sponge develops
no direct suction effect
on the intestinal wall. Neither are the typical sponge- and suction-caused
mucosa and wound changes
observed here. In case of direct sponge contact with the mucosa, the mucosa
fits itself to the sponge
surface so that the mucosa adheres nub-like in the pores of the sponge.
In a different embodiment, sections of the fluid collection element are
provided with a surface seal for
closure of the open pores. The surface seal may be provided by an elastic
adhesive, which can be
applied to the surface of the sponge in liquid form or as a spray and cures
here elastically.
This assures that the sealed surface of the sponge does not exert any suction
effect on the mucosa or
wound surface abutting here. The sponge body then becomes attached by suction
to the tissue surface
across the surface of its open pores only. As result of the sealing, an
effective local vacuum can continue
to be built up. Appropriately targeted placement of the sponge body assures
that the vacuum suction
and the suction cup-like attachment of the sponge body by suction is
undertaken only in a circumscribed
tissue region. In this way, any potential tissue injury by the vacuum suction
that does not require
treatment is avoided. At the same time, the local vacuum suction can be
applied at the location in need
of therapy.
With a cylindrical sponge body to be inserted into an esophagus, sealing may
advantageously be
undertaken on a third or half of the surface over the entire length of the
sponge body. Depending on the
configuration of the sponge body, different patterns for surface sealing are
possible. Placing a partially
sealed sponge body into the esophagus can achieve that, between the sealed
sponge surface and the
adjacent mucosa that is not exposed to the vacuum suction, saliva secretions,
fluids can even empty into
the stomach physiologically along the esophagus. Saliva retention is reduced,
a liquid diet can be made
possible. Along the seal, a feeding tube for enteral feeding can also be
installed.
Alternatively, the surface sealing can be produced using elastic films glued
onto the sponge.
Advantageously, these films may be longitudinally profiled so that secretions
can better drain along the
film by capillary action in a distal direction. Surface sealing may also be
implemented using longitudinally
halved elastic tubes which are attached to the sponge body by their convex
side by gluing. With the
mucosa abutting the concave side, a tubular tunnel is produced, through which
secretions can drain,
without being aspirated by the sponge body. The stated different types of
surface sealing may be
combined with each other.
Furthermore, in one embodiment, for passing through secretions, at least one
tubular tube is integrated
in the sponge body. This allows a flow of secretions (e.g. flow of saliva to
the stomach) through the
sponge body subject to vacuum suction. Premature clogging of the sponge pores
by viscous secretions is
prevented or delayed, so that the vacuum can develop its effect at the wound
bed or the mucosa better
and for a longer period of time. At the same time, in a treatment at the
esophagus, saliva retention can
be prevented and enteral nutrition made possible in intraluminal vacuum
therapy. Numerous new
therapeutic possibilities arise from the use of this embodiment.
As a particular advantage, the fluid collection element should be provided
with an additional complete
channel in the longitudinal direction. Through this channel, another sensor
can be introduced. It may be
17
CA 02829512 2013-09-09
particularly advantageous to introduce a tubular tube, which passes through
the entire length of the
collection element and projects beyond it by the ends. In a different
embodiment, the tube has the
same length as the fluid collection element, typically a sponge body. It is
not fluid-conductive and not
connected to the sponge body. Both at the proximal end and the distal end, it
may be provided with a
tulip-shaped flare. The tube does not collapse when the vacuum is applied,
hence is negative pressure
resistant. The tube is flexible, without breaking off as a result of kinking.
The tube serves as fluid pass-
through element for viscous secretions, such as saliva or feces. If these
secretions are passed through
the sponge body which is subject to vacuum suction, clogging of the sponge
pores will be prevented; at
the same time, the vacuum application to the wound bed can be maintained. Into
the fluid pass-through
element, sensors, endoscopic instruments, a guidewire or an elastic
installation and placement rod can
also be introduced. In particular, an endoscope can likewise be introduced. In
particular, an endoscope
can also be used as a guide element for installing a vacuum system with fluid
pass-through element. The
fact that the endoscope per se can be used as a guide rail for sponge drainage
greatly simplifies the
maneuver; full endoscopic control and visibility are gained and work steps are
saved in the placement of
the fluid collection element. The endoscope need not be removed from the body.
For this purpose, the
endoscope preferably has a diameter between 5 mm and 10 mm.
It has been found to be particularly advantageous that in this type of design,
when used in the
esophagus, sealing succeeds by particularly intimate, completely circular
suction over the entire length
of the sponge and that, as a result, very good and reliable coverage of a
defect in the esophagus with
simultaneous effective drainage at the wound bed is safely possible.
Advantageously, at the same time,
physiological oral enteral feeding is possible using this embodiment. These
are obvious advantages over
stenting alone, practiced in the prior art, with self-expanding covered
stents, that is to achieve the
defect coverage via an expansion force toward the outside.
The tube may be fixed in the sponge body by means of a suture, gluing or in
another manner. But no
special attachment of the tube within the channel of the fluid collection
medium is necessary. On the
contrary, if no attachment is carried out, this is especially advantageous.
Because, in that case, in a
removal maneuver, the tube can be easily removed from it, independent of the
sponge body. This is
particularly advantageous when the sponge body adheres very firmly to the
intestinal wall and is
mechanically detached from the wall using an endoscope. With suction applied,
the tube is fixed by
vacuum suction in the sponge body.
For placement of a vacuum drainage system equipped with such a fluid pass-
through element (i.e. the
sponge drainage unit, possibly including the overtube,), a pusher may be used.
The pusher has a tube,
into which an installation and placement rod or an endoscope can also be
introduced. The pusher can be
moved sliding on these guide elements. Using the pusher, a vacuum drainage
system can be moved
toward the distal end and can, as a result, be separated from the guide
element at the placement site.
Like the vacuum drainage system, the pusher is advantageously provided with a
longitudinal slot so that,
at any time during an examination, they can be placed laterally onto an
endoscope or removed.
Advantageously, the distal end and the proximal end of the fluid pass-through
element are radially
divided and are movable outward hinge-like or wing-like outward with respect
to a central tube section
of the fluid pass-through element. During movement on the guide element using
the pusher, all sections
of the fluid pass-through elements abut it. When vacuum suction is applied to
the sponge body, the
sponge body collapses, contracts and attaches itself to the intestinal wall by
suction. At the same time,
as a result, the movable ends of the fluid collection element unfold hinge-
like and spread open tulip-like.
18
CA 02829512 2013-09-09
Thereby, additionally, for adhering by suction, the vacuum drainage device
becomes anchored at the
placement site in a proximal and distal direction. As a result of the tulip-
like spreading, saliva and/or
secretions can accumulate more easily in the fluid pass-through element and
can be passed through the
fluid collection element, without being aspirated. This embodiment can be
applied particularly
advantageously for passing through physiologically accumulating secretions,
such as (depending on the
application site) saliva, small intestine or large intestine feces or air.
Advantageously, compared to
complete closure of the intestinal lumen by the vacuum therapy, using this
embodiment, in the case of
esophagus treatment simultaneous with the vacuum therapy, physiological oral
enteral feeding and/or
the insertion of feeding or stomach relief sensors is possible. In a treatment
involving the colon, this
allows feces to be evacuated and the installation of an artificial anus to be
avoided.
The ends of the fluid pass-through element can also consist of an elastic film
or other surface seals.
As an alternative to using the tube, a channel located in the sponge body can
be equipped with a surface
seal. This internal surface seal is advantageously made of a longitudinally
profiled film, along which
secretions are also preferably drained by capillary action, in this way
preventing clogging of the sponge
body in the intestinal wall contact area. Advantageously the surface seal
extends to the proximal end
and the distal end of the sponge body.
In one embodiment, the overtube forms a flexible plastic sleeve fitted to the
length of the endoscope in
the direction from proximal to distal (hereinafter the longitudinal
direction), into which the endoscope
can be inserted. For vacuum endoscopy, the length will advantageously be
selected in such a way that
the overtube is approximately 20 cm - 80 cm shorter than the endoscope. Over
this difference in length,
both can be moved back and forth relative to each other in the longitudinal
direction. The overtube can
be designed with different lengths and diameters. Moreover, it is
advantageously designed of a material
that allows individually adapting its length to the length of the endoscope,
e.g. by cutting if off at the
proximal end and/or the distal end. Preferably, the overtube is between 80 cm
and 160 cm long. But
other lengths are also possible.
The inside diameter of the overtube is preferably only slightly wider than the
outside diameter of the
endoscope, so that both can be easily moved relative to each other and the
overall diameter does not
become too large. Preferably, the inside diameter will be 8 mm to 15 mm wide,
but other inside
diameters are also possible. Preferably, the outside diameter is 10 mm to 25
mm wide, but other
outside diameters are also possible.
For better sliding, a lubricant can be used. Preferably, the outer sleeve of
the endoscope, the inside and
the outside of the overtube are coated using a low-friction, especially
additionally hydrophilic material.
Advantageously, the proximal end of the overtube has a funnel-shaped
enlargement so that an
endoscope can be more easily inserted. Advantageously, at the proximal end of
the overtube, a valve-
like closure is provided, through which insertion of an endoscope is possible,
escape of examination gas
or secretions is prevented. Advantageously the lumen is tapered at the distal
end, so that it abuts the
endoscope and, as a result, prevents gradation, which would make pushing the
entire unit forward
difficult or rather facilitates sliding relative to the endoscope.
In one embodiment, the overtube has, immediately proximal and distal relative
to the imposed fluid
collection element, an annular lip-like thickening, so that during suction
build-up proximally and distally
19
CA 02829512 2013-09-09
relative to the sponge, at the connection of the lip to the intestinal wall,
an intimate connection and
thus a better seal is created, which facilitates vacuum build-up at the
sponge. Preferably, the annular
swells are produced elastic. Preferably, the swells are also slotted like the
overtube.
One embodiment of the sponge drainage device has a support sleeve. It is
designed in such a way that it
can be mounted on top of the overtube and/or the endoscope and removed again.
It is particularly
designed in such a way that, fluid-conducting, it connects the drainage hose
that is situated in the
overtube and/or the endoscope and the fluid collection element, hence the
sponge of the sponge
drainage device. Preferably, the support sleeve is attachable, together with
the fluid collection element,
on the overtube/the endoscope by means of gluing, adhesive tape, elastic,
string or any other fastening
option above the suction ports or is already attached accordingly during their
production. The use of the
overtube/endoscope is, however, optionally possible, depending on the
application, with or without
fluid collection element.
The overtube and the imposed fluid collection element and the support sleeve
are preferably
longitudinally slotted over their entire lengths. The longitudinal slot offers
the advantage that, at any
moment during an endoscopic examination, the overtube can be attached to an
endoscope and also
removed again. This slot can be closed by gluing, adhesive tape, string,
zipper or any other technical
means. The closing mechanism is preferably designed in such a way that it can
be repeatedly opened
and closed.
As a particular preference, the support sleeve has, at its proximal and its
distal end, annular swells. An
annular lip can also be attached to the proximal and/or distal end of the
fluid collection element.
Preferably, this lip is attached to the fluid collection element by gluing.
Preferably, the annular lip-like
swell is created by stable compression and adhesion of the fluid collection
medium. The fluid collection
element is placeable on the overtube from the side.
In a particular embodiment, the fluid collection element consists of an open-
pore thin fluid-conductive
film. It has the particular advantage that the diameter of the overtube in the
area of the fluid collection
element is not substantially increased and that, as a result, the overtube can
slide freely. It is, however,
important to assure that the open-pore film can be fluid-conductive and
forward the suction to be
applied unabated, so that the fluid collection element becomes attached by
suction and is fixed in place.
In a variant, the overtube is designed so as to receive a plurality of fluid
collection elements and
drainage hoses in different longitudinal sections. This advantageously assures
that the anchoring of the
overtube not only takes place at the distal end, but also in other locations
along the overtube, too.
In or on the overtube, other working channels may be provided, which extend
longitudinally from
proximal to distal inside the overtube. Like the overtube, they may also be
designed longitudinally
slotted for opening and closing. These working channels can be used for
flushing, aspirating or inserting
instruments.
Overtube as well as endoscope are preferably provided with measurement
markings so that, on the one
hand, the penetration depth can be determined, but on the other hand, it is
possible to measure in how
far both are displaced relative to each other.
CA 02829512 2013-09-09
Vacuum enteroscopy can be carried out using conventional endoscopes. When
using a conventional
endoscope with a vacuum sponge overtube, only the vacuum anchor is used by the
vacuum on the fluid
collection medium of the overtube.
The length of the overtube should be selected shorter than that of the
endoscope so that mobility
relative to the endoscope is possible. Preferably, the endoscope has a length
between 120 cm and 220
cm, but other lengths are also possible. Preferably, the endoscope has an
outside diameter of 8 cm to 12
mm. But other outside diameters are also possible.
For using the vacuum above a sponge drainage device on the endoscope,
specially designed endoscopes
are required, which are described below:
Integrated in the endoscope are preferably one or more fluid communication
elements. They are
preferably designed as negative pressure-resistant plastic channels in a wall
of the endoscope, which, as
a special preference, are fluid-conductive and perforate the outer sleeve of
the endoscope at the distal
end of the endoscope with a perforation opening or a plurality thereof and
terminate here. These
negative pressure-resistant suction channels are connected to the vacuum pump
via negative pressure-
resistant fluid-conducting connections, so that fluids and gas can be drained
by suction. The fluid
communication element (the channel) in the endoscope is preferably
cylindrical. Preferably, the channel
is arranged parallel to a longitudinal axis of the fluid collection element.
Such an endoscope can be used with or without any fluid collection element.
With the special
endoscope, conventional examinations can be performed, too.
When using a fluid collection element, preferably attached at the level the
openings in the fluid
communication elements, it is attached by gluing, string, clamping or any
alternative fastening
possibility. Preferably, the channel of the fluid collection element
encompasses the entire circumference
of the endoscope at the level of the openings of the fluid communication
element. The fluid collection
element can also be only partially encompass the endoscope. In a special
embodiment, the fluid
collection element consists of an open-pore thin fluid-conducting film. It has
the special advantage that
the diameter of the endoscope in the area of the fluid collection element is
not substantially larger and
that, as a result, the endoscope can slide unimpeded. It is important to
assure that the open-pore
coating can direct the suction to be applied and is fluid-conductive, so that
the fluid collection element
becomes attached by suction and is fixed in place.
Preferably, the longitudinal axis of the fluid collection element
substantially coincides with the
longitudinal axis of the endoscope or is at least parallel to it. Furthermore,
the channel is preferably
arranged parallel to an axis of symmetry of the fluid collection element.
Via working channels, endoscopic instruments in the endoscope can be guided to
the distal end of the
endoscope. Using these instruments, surgical procedures, such as a tissue
resection, can be performed
under endoscopic vision. The endoscope may for example have one or 2 working
channels. As a result of
the arrangement within the endoscope, these inner working channels have very
small sizes, in order to
achieve the smallest possible device diameter for the endoscope.
21
CA 02829512 2013-09-09
A preferred embodiment provides for a guide sleeve, which is attached to the
endoscope, at its distal
end, for instance, and provides an additional external insertion accessory for
endoscopic instruments or
accessories and/or a flushing and aspiration channel. The sleeve is a
dimensionally stable hose or a
tubular structure, which does not collapse or break off as a result of
kinking. It is flexible, in order to
allow following the movements of the endoscope. Another advantage over the
internally located guide
channels is the fact that an outer guide channel may have a larger diameter.
With this guide sleeve, the
endoscope is equipped with additional outer working channels, which allows
extending the endoscopic
treatment options.
At its proximal end, for instance, the guide sleeve may be sealed by a valve,
in order to prevent the
escape of an examination gas. In varying embodiments, the guide sleeve allows
simultaneous
attachment of an outer guide channel or a plurality thereof. It may be
produced with different
diameters.
The fastening accessory on the endoscope may be designed in the form of a
sleeve encompassing the
endoscope, elastic, adhesive tape or any other type of fastening device. The
fastening accessory can be
designed in such a way that even removal of the external guide accessory would
be possible in the
inserted endoscope.
New endscopic treatment options result from the possibility of removability of
the guide sleeve. The
outer insertion accessory can, for instance, also be used for pushing forward
a guidewire for other
endoscopic accessories. After placement of the guidewire, the outer working
channel can be removed
and, for instance, a stent can be introduced via the guidewire subject to
optical monitoring of the
endoscope that is in place within. The endoscope need not be removed to
perform the procedure. In a
special embodiment of the vacuum drainage device (in analogy to the inner
working channel of the
endoscope), it can instead be directly inserted through the lumen of the outer
working channel at the
placement site.
Advantageously, the lumen of the outer working channel is wider than in the
case of an inner working
channel, so that, utilizing the advantages of direct endoscopic guidance, a
vacuum drainage device may
be more bulky.
In a special embodiment, the working channel is distally provided with lateral
perforation openings and
connected to a fluid collection element and can thus, by itself, be used as a
sponge drainage device.
The insertion sleeve may also be produced longitudinally slotted. This allows
for an instrument inserted
through the sleeve, with the endoscope horizontal, to be laterally released
from the sleeve and
additional removable instruments could be inserted via the slotted insertion
accessory.
Hereinafter, exemplary embodiments are described, which enhance the negative
pressure probe.
Within the framework of the application herein, it is also referred to as
sensor, with the same meaning.
The sensor can be applied in both the vacuum therapy sponge therapy on
external visible wounds and
on intracorporeal wounds which are not visible from the outside, in order to
measure the vacuum that is
actually being applied to the wound. The sensor can be inserted both in wound
treatment using the
fluid collection element.
22
CA 02829512 2013-09-09
The sensor one sensors are connected, either wired or wireless, to the
pressure regulating unit of the
vacuum system, in one variant directly connected to the vacuum pump, so that
preset required negative
pump pressure values to be generated can be monitored and regulated.
Especially in varied
embodiments, the sensor can be placed on the polyurethane sponge, be applied
abutting the sponge, or
between sponge and drainage. But it may instead be arranged within the fluid-
conducting system of the
drainage hose.
Preferably, a plurality of sensors exist for measuring the generated negative
pressure. If multiple sensors
exist, they can also perform measurements at different locations and transmit
them, for example at the
negative pressure side pump output, in the secretion container, at the fluid
collection element or in a
fluid communication element.
In one embodiment, at least one of the sensors is permanently integrated in
the pump system.
Alternatively or additionally, at least one of the sensors is designed so as
to be retroactively
introducible, for example into a fluid communication element.
In this case, the drainage unit can be designed in such a way that the sensor
is integrated in the system
from the start, but it can instead be retroactively applied to/in the fluid
collection element, after the
fluid collection element has been inserted into the wound. For this purpose,
it can be conducted all the
way to the fluid collection element or the wound inside the fluid
communication element, or it can be
conducted to the wound site separately in a second fluid communication
element. It is also possible to
equip the fluid communication element with a second lumen for this purpose. In
doing so, it is
advantageous if the sensor is designed in the form of a wire in such a way
that, like an endoscopic
control mandarin, it can be easily pushed forward even in a small lumen.
Preferred embodiments according to the invention are described hereinafter
with reference to figures.
Additional preferred embodiments according to the invention will be explained
hereinafter with
reference to the accompanying figures according to their structure and
handling.
Figure 1a is a schematic representation of an exemplary embodiment of a vacuum
system;
Figure lb is a block diagram with further details of the pressure regulating
unit of the vacuum system of
Figure la;
Figure 2 is a partial longitudinal section of the vacuum system of Figure la;
Figure 3 is a schematic representation of another exemplary embodiment of a
vacuum system;
Figure 4 is a schematic representation of an arrangement of a fluid collection
element;
Figure 5 is a schematic partial longitudinal section of the arrangement of
Figure 4;
Figure 6 is a longitudinal section of a fluid collection element 64, which is
connected, fluid conducting, to
two fluid communication elements 63,
23
CA 02829512 2013-09-09
Figure 7 is a longitudinal section of a fluid collection element, in which
both a fluid conducting fluid
communication element and imposed on it, a wire-like negative pressure sensor
is arranged.
Figure 8 shows an embodiment of a longitudinally slotted overtube;
Figure 9 is a longitudinal section of Figure 8;
Figure 10 is a cross-section of an overtube;
Figure 11 is a cross-section of a different variant of an overtube;
Figure 12 shows an additional embodiment of an overtube;
Figure 13 is a cross-section of the overtube of Figure 12;
Figure 14 is a different representation of the embodiment of Figures 12 and
13;
Figure 15 is a longitudinal representation of Figure 14;
Figure 16 is a representation of an overtube, which forms a variant of the
overtube of Figures 12 to 15;
Figure 17 is a representation of a different variant of an overtube;
Figure 18 is a longitudinal section of the overtube of Figure 17;
Figure 19 shows a variant of the representations of the embodiments of Figure
17 and Figure 18;
Figure 20 is a longitudinal section of the overtube Figures 18 and 19;
Figure 21 is an additional longitudinal section of the overtube of Figures 18
to 20;
Figure 22 is a representation of a distal end of an endoscope;
Figure 23 is a longitudinal section of the endoscope of Figure 22;
Figure 24 is an additional longitudinal section of the endoscope of Figure 22;
Figure 25 is a representation of a fluid collection element suitable for use
on the overtube, the
endoscope and the support sleeve;
Figure 26 is a longitudinal section of the fluid collection element of Figure
25;
Figure 27 is a representation of a different fluid collection element;
Figure 28 is a longitudinal section of Figure 27;
Figure 29 is a representation of a support sleeve for a fluid collection
element;
24
CA 02829512 2013-09-09
Figure 30 is a longitudinal section of the support sleeve of Figure 29;
Figure 31 is a representation of a support sleeve having, attached on it
between lip-like rings, a
longitudinally slotted fluid collection element;
Figure 32 is a longitudinal section of the support sleeve Figure 31;
Figures 33 a-i show different variants of cross-sectional profiles of lip-like
ring closures;
Figure 34 is a representation for explaining, how a flexible endoscope is
inserted or removed via the
longitudinal slot of the overtube;
Figure 35 shows an endoscopy arrangement according to a different exemplary
embodiment;
Figures 36 a-n show a schematic representation of the examination process of a
video endoscopy
treatment;
Figure 37 is a representation of a vacuum drainage with partial surface
sealing of the sponge body;
Figure 38 is a longitudinal section of the fluid collection element of Figure
37;
Figure 39 is a representation of a different embodiment of a vacuum drainage;
Figure 40 is a longitudinal section of the vacuum drainage of Figure 39;
Figure 41 is a representation of a vacuum drainage with a profiled surface
seal;
Figure 42 is a cross section of the vacuum drainage of Figure 41;
Figure 43 is a representation of a vacuum drainage with a tube attached in a
sponge body;
Figure 44 is a representation of a different embodiment of a vacuum drainage
having a tube attached in
a sponge body;
Figure 45 is a longitudinal section of the vacuum drainage of Figure 43;
Figure 46 is a representation of an additional embodiment of a vacuum drainage
having a drainage hose
in a sponge body;
Figure 47 is a longitudinal section of an additional vacuum drainage having a
tube situated in the sponge
body;
Figure 48 is a representation of the vacuum drainage of Figure 47, in this
representation, a negative
pressure being applied to the drainage hose;
CA 02829512 2013-09-09
Figure 49 is a representation of an additional embodiment of a sponge
drainage;
Figure 50 is a longitudinal section of the sponge drainage of Figure 49;
Figure 51 is a representation of an additional embodiment of a sponge
drainage;
Figure 52 is a longitudinal section of the sponge drainage of Figure 51;
Figure 53 is a representation of an additional embodiment of a sponge
drainage;
Figure 54 is a longitudinal section of the sponge drainage of Figure 53;
Figures 55 a to h show different variants of a distal end of a sponge
drainage, each in a longitudinal
section.
Figures 56 a to f are different representations of a drainage hose and pointed
top-seated attachments;
Figures 57 a to fare different representations of an endoscopic insertion
instrument;
Figures 58 a to e are different representations of an additional endoscopic
insertion instrument;
Figure 59 is a representation of insertion accessory with a sleeve for
attachment to a distal end of an
endoscope;
Figure 60 is a representation of two different different-size insertion
accessories;
Figure 61 shows a cross section of an insertion accessory and of an attachment
sleeve with a valve;
Figure 62 shows a representation of an insertion accessory with an attachment
sleeve on a distal end of
an endoscope; and
Figure 63 is a representation of an insertion accessory with an attachment
sleeve on a distal end of an
endoscope.
Figure la is a schematic representation of an exemplary embodiment of a vacuum
system having a
vacuum pump 11, a secretion container 12 on the pump, a fluid communication
element 13, which leads
from the vacuum pump to a fluid collection element 14. Into the fluid
communication element, via a
lateral input 15, a negative pressure sensor 16 is introduced, which
electronically transmits to the
vacuum pump, via a pressure regulating unit 17, measured values for adjusting,
presetting and
controlling via connecting elements 18. Connected to the pressure regulating
unit but not illustrated
here in detail (but compare Figure lb), is a user input unit. The pressure
regulating unit 17 has a test
signal input for receiving test signals from negative pressure sensor 16. The
latter is designed to control
the vacuum pump 11 during operation for generating and maintaining a vacuum at
the hollow space to
be treated at a predetermined negative pressure of, in this example, between
60 mm Hg and 500 mm
Hg, within a predetermined evacuation period between 0.5 and 5 seconds. For
this purpose, the vacuum
pump 11 has a control input 11.1.
26
CA 02829512 2013-09-09
Figure lb shows a simplified block diagram with further details of the
pressure regulating unit 17 of the
vacuum system of Figure la. The pressure regulating unit 17 has a control unit
17.1 implemented as a
programmable microprocessor or a microcontroller or a special integrated
circuit (ASIC). The control
unit receives test signals generated by the negative pressure sensor 16.
Furthermore, it is with the user
input unit Ul . Via the user input unit Ul, the physician can input
parameters, such as a negative pressure
to be set, an evacuation period and a potentially present dead volume. This
input need not necessarily
take the form of specific values. It may alternatively or additionally, for
instance, instead be intended,
via user input unit Ul, to identify a predefined therapy or examination type
by menu selection or text
input, for which purpose, in a memory 17.2 of the pressure regulating unit,
predefined negative
pressure parameters (if applicable, of its development over time) and the
evacuation period are stored
and can be called up via the input. The dead volume that may have to be taken
into account for
determining a suction capacity of the connected vacuum pump 11 can be either
input quasi
automatically by user input, alternatively instead by reading in a code. The
pressure regulating unit is
designed to determine the required suction capacity of the pump using the
negative pressure value on
the hollow space to be treated, which (value) is selectable from a predefined
negative pressure value
interval (automatic value monitoring for reliability after input, using
prestored threshold values) and an
evacuation period, the value between 0.5 and 5 seconds of which is selectable.
Depending on the
situation additional parameters are taken into account:
i) figuring in a predetermined dead volume of the vacuum drainage
arrangement that is connectable to the vacuum pump, to determine a first
suction
capacity of the vacuum pump, required for generating the specified negative
pressure at
the hollow space to be treated within the specified evacuation period, and to
transmit a
corresponding first control signal to the control input 11.1 of the vacuum
pump 11,
ii) upon generating the specified negative pressure at the hollow space to be
treated, to monitor the pressure test signal and to determine, as a function
of the
current pressure test signal, a second suction capacity of the vacuum pump,
required for
maintaining the specified negative pressure, and to transmit a corresponding
second
control signal to the control input 11.1 of the vacuum pump 11; and
iii) upon generating the specified negative pressure at the hollow space to be
treated, if a deviation of the measured pressure or negative pressure from the
specified
negative pressure exists that exceeds a predefined threshold of the measured
pressure
or negative pressure, to determine a third suction capacity that is required
for
generating the specified negative pressure within the specified evacuation
period and to
transmit an appropriate third control signal to the control input 11.1 of the
vacuum
pump 11.
In the cases ii) and iii), the dead volume must, as a principle, be taken into
account as well. It can be
neglected for mere maintenance of a vacuum in a variant. In case iii) it must,
however, preferably be
taken into account.
Via a switch S. which may even be directly integrated into the user input unit
Ul, it is possible to switch
from an endoscopy mode to a therapy mode and back. The difference between the
modes lies in the
27
CA 02829512 2013-09-09
range of values available for the negative pressure. No patient should be
exposed to high negative
pressure values in the therapy mode without a physician present. Such higher
negative pressure values
are, therefore, only available in the endoscopy mode. Another difference lies
in the input options via the
user input unit Ul. They are limited in the therapy mode, so that the patient
cannot make any
undesirable, harmful parameter changes. The switch is secured by a key and can
only be activated by
the treating physician.
Figure 2 is a partial longitudinal section of the vacuum system of Figure 1.
Into the fluid communication
element, via a lateral input 15, the negative pressure sensor 16 is inserted,
which, via the pressure
regulating unit 17, transmits the measured values for regulating, preadjusting
and controlling to the
vacuum pump by means of connecting elements 18.
Figure 3 is a schematic representation of a different exemplary embodiment of
a vacuum system with a
presecretion container 39 for faster suction build-up and with secretion
container 32. The presecretion
container is connected to the secretion container via a filter/valve 310. The
pressure regulating unit 37
for the negative pressure values, time settings, evacuation periods and for
alarm functions is connected
to vacuum pump 31 by means of connecting elements 38. A fluid collection
element 34 is connected to
the pump unit by means of a fluid communication element 33.
Figure 4 is a schematic representation of an arrangement of a fluid collection
element 44, which is fluid-
conducting and connected to a fluid communication element 43. Into the fluid
communication element,
via a lateral input, through a valve 411, a wire-like negative pressure sensor
46 has been pushed forward
up to the fluid collection element 44. The negative pressure sensor is
connected to a measuring and
pressure regulating unit 47, which can forward the test signals of the
negative pressure sensor via an
electronic connection 48.
Figure 5 is a schematic partial longitudinal section of the arrangement of
Figure 4. The fluid collection
element 44 is connected to the fluid communication element 43, at the distal
end of which fluid-
conducting openings 412 exist for suction. Into the fluid communication
element, a wire-like negative
pressure sensor 46 has been advanced up to the fluid collection element. At
the distal end of negative
pressure test probe 413 of the sensor is attached. The test probe is connected
to a measuring and
pressure regulating unit 47, which can forward the information via an electric
connection 48.
Figure 6 is a longitudinal section of a fluid collection element 64, which is
fluid-conducting and
connected to two fluid communication elements 63 into one of the fluid
communication elements, a
wire-like negative pressure measuring sensor 66 has been advanced up to the
fluid collection element.
At its distal end, a negative pressure sensor 613 is attached. Another
negative pressure sensor 613a
exists in the fluid collection medium.
Figure 7 is a longitudinal section of a fluid collection element 74, in which
is arranged both, a fluid-
conducting fluid communication element 73 and, imposed on it, a wire-like
negative pressure sensor 76.
The negative pressure sensor 76 is connected to a pressure regulating unit 77
and is equipped, at its
distal end, with a negative pressure sensor 713 which is located in the fluid
collection element. The
pressure regulating unit is enhanced by an alarm function. Electronic control
signals are transmitted for
regulation of the negative pressure, in particular to the vacuum pump. Alarms
regarding a malfunction
can be triggered.
28
CA 02829512 2013-09-09
Figure 8 is a representation showing an embodiment of a longitudinally slotted
overtube 81. At its distal
end, overtube 81 is conically tapered to prevent injury during insertion. Over
the entire length, a
complete slot 86V exists. At its proximal end 83, overtube 81 is designed
funnel-shaped to facilitate
insertion of an endoscope. The overtube is provided with a fluid communication
element 84V in the
form of a drainage line, which is integrated in the wall and extends from
proximal to distal. It ends at the
distal end in lateral openings 85V and perforates the wall of the overtube by
means of them. At the
proximal end, it exits hose-like (84V) and can be connected here to the vacuum
device.
Figure 9 is a longitudinal section of the overtube 81 of Figure 8, including
representation of overtube 81,
which tapers at the distal end 82, widens funnel-shaped at the proximal end
83, and includes fluid
communication element 84V, which ends at its distal end in fluid-conducting
wall openings 85V, and is
conducted out hose-like from the wall.
Figure 10 is a cross-section of a different exemplary embodiment of an
overtube 101 with a fluid
communication element 104V integrated in the wall. The overtube 101 is shown
with a longitudinal slot
106V.
Figure 11 is a cross-section of a different variant of an overtube 111,
having, integrated in the wall, a
fluid communication element 114V, which is fluid-conducting and perforates the
wall by means of an
opening 115V and is fluid-conducting and connected to the outside wall of
overtube 111. The cross -
section is drawn at the level of wall opening 115V. The overtube is
represented with a longitudinal slot
116V.
Figure 12 is a representation showing an embodiment of an overtube 121. Over
entire length, a
longitudinal slot 126V exists. Overtube 121 is equipped with a fluid
communication element 124V in the
form of a working channel integrated in the wall and extending from a proximal
wall opening 127 of the
overtube to the distal tip and ends here with a distal wall opening 128.
Figure 13 is a cross-section of the overtube 121 of Figure 12, the channel-
type fluid communication
element 124V being provided with a longitudinal slot 1210. The fluid
communication element is
integrated into the wall of overtube 121. The overtube is also represented
with the longitudinal slot
126V.
Figure 14 is a different representation of the embodiment of Figures 12 and
13, into the fluid
communication element 124V, via the proximal opening 127, a medical instrument
1220, herein a
guidewire, having been introduced and conducted out through it via the distal
opening 128.
Figure 15 is a longitudinal section of overtube 121 which shows the working
channel 129 that is
integrated into the wall of overtube 121 as well as the proximal wall opening
127 and the distal wall
opening 128.
Figure 16 is a representation of an overtube 161 that embodies a variant of
the overtube of Figures 12
to 15. A working channel integrated into the wall of overtube 161 has, over
the entire length between
proximal wall opening 167 and distal wall opening 168, a longitudinal slot
1610. Overtube 161 also has a
longitudinal slot 166V over the entire length in this embodiment.
29
CA 02829512 2013-09-09
Figure 17 is a representation of an overtube 171, over the entire length of
which a complete slot 176V
exists. Overtube 171 is equipped with two fluid communication elements 174V in
the form of drainage
lines, which are integrated into the wall of the overtube. They end in lateral
openings 175V at the distal
end of the overtube. The proximal ends 1711 of the fluid communication
elements are fluid-conducting
and connected to the vacuum unit. Attached to the overtube, proximal and
distal relative to the lateral
openings 175V of the fluid communication elements are annular lip-like swells
1712V.
Figure 18 is a longitudinal section of overtube 171 of Figure 17, which shows
the two fluid
communication elements 174V, which are fluid-conducting and end laterally in
the distal end of the
overtube with openings 175V. Proximal and distal of these, the annular lip-
shaped swells 1712V are
attached to the overtube.
19 shows a variant of the representations of the embodiments of Figure 17 and
Figure 18, here, at the
level of the distal wall openings 175V, between the annular swells 1712V,
additionally, the fluid
collection element 1713V being attached. The fluid collection element is also
provided with a
longitudinal slot 176V on the longitudinal axis of overtube 171.
Figure 20 is a longitudinal section of overtube 171 of Figures 18 and 19,
which shows clearly the fluid
communication elements 174V with the fluid-conducting wall openings 175V,
fluid collection element
1713V attached above and proximal and distal lip-like swells 1712V.
Figure 21 is an additional longitudinal section of the overtube of Figures 18
to 20, which shows the fluid
communication elements 174V, with the fluid-conducting wall openings 175V and
the proximal and
distal lip-like swells 1712V. In addition, a wire-like measuring sensor 1719
is represented, which was
introduced into one of the fluid communication elements and which ends
distally in a negative pressure
measuring unit 1721. The measuring sensor 1719 has been introduced into the
fluid communication
element 174V via a valve 1722.
Figure 22 is a representation of a distal end of an endoscope 2214. At the
distal end of endoscope 2214,
lateral fluid-conducting wall openings 225E of a fluid communication element
incorporated in the
endoscope are represented. Proximal and distal relative to these wall openings
225E, lip-like rings 2212E
are attached to endoscope 2214.
Figure 23 is a longitudinal section of endoscope 2214 of Figure 22 showing the
internally-situated fluid
communication element 224E, the lip-like rings 2212E proximal and distal
relative to the lateral
openings 225E of fluid communication element 224E.
Figure 24 is an additional longitudinal section of endoscope 2214 of Figure
22. In this representation,
above the fluid-conducting openings of fluid communication element 224E,
between the lip-like rings
2212E, a fluid collection element 2213E is inserted.
Figure 25 is a representation of a fluid collection element 2513V, 2513E,
2513T, which is suitable for use
on the overtube, the endoscope and the support sleeve. In this embodiment, the
fluid collection
element has, at its ends, a conical taper 2515. A channel 2516 is arranged
centrally along the
longitudinal axis of the fluid collection element.
CA 02829512 2013-09-09
Figure 26 is a longitudinal section of fluid collection element 2513V, 2513E,
2513T of Figure 25. The
conical taper 2515 can be recognized at the ends and at the central channel
2516 along the longitudinal
axis.
Figure 27 is a representation of another fluid collection element 2713V,
2713E, 2713T for overtube,
endoscope and support sleeve, a lip-like ring 2712V, 2712E, 27121 being
attached to each end of the
element. A joint central channel 2716 extends through the fluid collection
element.
Figure 28 is a longitudinal section of Figure 27 with fluid collection element
2713, 2713E, 2713T, a lip-
like ring (2712V, 2712E, 2712T) being attached to each end.
Figure 29 is a representation of a support sleeve 2917 for a fluid collection
element, shown with a
longitudinal slot 296T, fluid-conducting wall perforations 295T and lip-like
rings 2912ST proximal and
distal relative to the wall perforations 295T. The rings are also slotted.
Figure 30 is a longitudinal section of the support sleeve of Figure 29 and
shows the fluid-conducting wall
perforations 295T and the lip-like rings 2912T proximal and distal relative to
the wall perforations 2951.
Figure 31 is a representation of a support sleeve 3117 having, attached on it,
between lip-like rings
31121, a longitudinally slotted fluid collection element 31131. Wall
perforations 315 of the support
sleeve are indicated by dashed lines.
Figure 32 is a longitudinal section of the support sleeve of Figure 31, on
support sleeve 3117, between
the lip-like rings 3112T and fluid-conducting with the wall perforations 315T,
fluid collection element
3113T being attached.
Figures 33 a-i show different variants of cross-sectional profiles of the lip-
like ring closures 3112V,
3112E, 3112T, which are mounted to an exterior wall 3118V, 3118E, 3118T of
overtube, endoscope or
support sleeve.
Figure 34 shows a flexible endoscope 3414 in a state, in which it is
introduced and removed via
longitudinal slot 346V of an overtube 341. To the endoscope and the overtube,
fluid collection elements
3413E, 3413V are mounted, in each case at the distal end. The fluid
communication element 344V is
connected fluid-conductive to the fluid collection element of overtube 3413V.
Figure 35 shows an endoscopy arrangement according to an additional exemplary
embodiment.
Represented is a vacuum pump unit 3521 having a secretion collection container
3522, to which an
overtube 351 and an endoscope 5614 are connected. To the distal ends of
overtube 351 and endoscope
3514, fluid collection elements 3513V and 3513E are attached, which are
connected to vacuum pump
unit 3521 via the fluid-communication elements 354V (overtube) and 354E
(endoscope).
Figure 36 a-n is a schematic representation of the examination process of a
vacuum endoscopy. The
treatment comprises the following steps:
a) Insertion of endoscope 3614 with fluid collection element 3613E into an
intestine 3625 of a patient;
31
CA 02829512 2013-09-09
b) Subsequent insertion of overtube 361 with fluid collection element 3613V
above the endoscope;
c) Subjecting fluid collection element 3613V to a vacuum;
d) Pushing endoscope 3614 forward in the intestine;
e) Subjecting fluid collection element 3613E on endoscope 3614 to a vacuum;
during this step, no
vacuum application to fluid collection element 3613V on overtube 361;
f) Pushing overtube 361 on above the attached endoscope 3614;
g) Subjecting both fluid collection elements 3613E and 3613V to a vacuum;
h) Optional straightening maneuver if necessary, by retracting the fluid
collection element 3613E
together with the fluid collection element 3613V, both subject to vacuum
application;
i) Disconnecting the vacuum application to fluid collection element 3613E
while maintaining the vacuum
application to fluid collection element 3613V;
j) Pushing endoscope 3614 forward while overtube 361 is held in place by the
vacuum;
k) Maintaining the vacuum application to fluid collection element 3613E,
disconnecting the vacuum
application to fluid collection element 3613V;
I) Pushing overtube 361 on with endoscope 3614 held in place by the vacuum;
m) Vacuum application to both fluid collection elements 3613E and 3613V;
n) Straightening maneuvers by retracting fluid collection element 3613E
together with fluid collection
element 3613V, both subject to the vacuum;
Thereafter, the examination can be continued using Step i) and following.
Figure 37 is a representation of a fluid collection element (a vacuum drainage
device) in the form of a
sponge body 371 with partial surface seal 374 of sponge body 371. Into a
drainage hose 372, which is
introduced into sponge body 371, a guidewire 373 is introduced in this
representation.
Figure 38 is a longitudinal section of fluid collection element 371 of Figure
37. The surface seal 374 of
sponge body 371 with drainage hose 372, which has lateral perforation openings
372a and into which a
guidewire 373 is introduced, are shown.
Figure 39 is a representation of a different embodiment of a vacuum drainage
device 391 with a
drainage hose 392 and a guidewire 393 situated therein. On the outside of
sponge body 391, a bowl-
shaped seal 395 is arranged, which has a funnel-shaped flare 395a at its
proximal end.
Figure 40 is a longitudinal section of the vacuum drainage device 391 of
Figure 39 and also shows the
bowl-shaped seal 395 on the outside of the sponge body of vacuum drainage
device 391 which is flared
32
CA 02829512 2013-09-09
funnel-like at the proximal end (395a). Also shown is drainage hose 392, which
has lateral perforation
openings 392a and in which a guidewire 393 is situated.
Figure 41 is a representation of a vacuum drainage device in the form of a
sponge body 411 having a
profiled surface seal 416 of sponge body 411. In drainage hose 412, a
guidewire 413 is introduced. The
surface seal 416 has a riffled profile 416a with longitudinal grooves running
side by side in the
longitudinal direction of sponge body 411.
Figure 42 is a cross section of the vacuum drainage device of Figure 41. In
drainage hose 412, guidewire
413 is situated. The surface seal shows its longitudinal profile 416a.
Figure 43 is a representation of a vacuum drainage device having a tube 437
attached in sponge body
431. Into sponge body 431, a drainage hose 432 is inserted, in which a
guidewire 433 is situated. At the
proximal end of the vacuum drainage device, a funnel-shaped flare 437a exists.
Into tube 437, an
insertion rod 438 is introduced, which is conically tapered at its distal end
438a. Into insertion rod 438,
an additional guidewire is inserted. At the proximal end of the insertion rod,
a pusher 439 is imposed.
Figure 44 is a representation of a different embodiment of a vacuum drainage
device having, attached in
a sponge body 441, a tube 447, which has a funnel-shaped flare at its proximal
end. In the tube, an
endoscope 4410 is introduced. On the proximal end of endoscope 4410, a pusher
449 is imposed. Tube
447, sponge body 441 and pusher 449 are provided with a complete lateral
longitudinal slot 4412. In
sponge body 4411, a drainage hose 442 is inserted; in it, a guidewire 443 is
situated.
Figure 45 is a longitudinal section of the vacuum drainage device of Figure
43. In sponge body 431 lies
tube 437, which has its funnel-like flare 437a at the proximal end. In tube
437, insertion rod 438 is
situated. In insertion rod 438, a guidewire 433 is introduced. On the
insertion rod, pusher 439 is
imposed. In sponge body 431 lies the drainage hose 432 with lateral openings
432a. In drainage hose
432 lies an additional guidewire 433a.
Figure 46 is a representation of an additional embodiment of a vacuum drainage
device with drainage
hose 462 in sponge body 461. In the sponge body of the vacuum drainage device,
a tube 467 is situated,
which has proximal and distally split ends 467b. Arrows indicate, in which
direction the split ends 467b
can open.
Figure 47 is a longitudinal section of an additional vacuum drainage device
having, situated in sponge
body 471, a tube which, subject to suction, can be opened outward by its ends
477b. Into tube 477, an
endoscope 4710 is introduced. In sponge body 471 lies a drainage hose 472 with
lateral openings 472a.
The vacuum drainage device is situated in a section of the intestine, of which
an intestinal wall 4713 is
indicated.
Figure 48 is a representation of the vacuum drainage device of Figure 47, in
this representation, a
negative pressure being applied to drainage hose 472. Sponge body 471 has,
therefore, collapsed and
intestinal wall 4713 abuts sponge body 471. Movable ends 477b of tube 477 are
folded outward in the
direction of the arrows.
33
CA 02829512 2013-09-09
Figure 49 is a representation of an additional embodiment of a vacuum drainage
(device), which is
identified as sponge drainage (device), with the same meaning, within the
framework of the application
herein. A sponge body 491 is attached to a drainage hose 492a. Drainage hose
492 exits proximally and
distally from the sponge body. Into drainage hose 492a, a guidewire 493 was
introduced.
Figure 50 is a cross-section of the sponge drainage device of Figure 49.
Sponge body 491 is attached on
drainage hose 492a above the perforation openings 494. A guidewire 493 is
introduced into the
drainage hose.
Figure 51 is a representation of an additional embodiment of a sponge drainage
device. Two sponge
bodies 511 are attached on a drainage hose 512a at a (certain) distance (from
each other). Into drainage
hose 512a, a guidewire 513 was introduced. This embodiment is advantageous,
if, for instance, a section
of the intestine is to be functionally disabled by means of a fistula.
Figure 52 is a cross-section of the sponge of the drainage device of Figure
51. The two sponge bodies,
attached at a distance (from each other) on drainage hose 512a above
perforation openings 514, are
recognizable. Guidewire 513 is introduced into the drainage hose.
Figure 53 is a representation of an additional embodiment of a sponge drainage
device. A sponge body
531 is attached on a drainage hose 532a. Drainage hose 532a tapers to form a
small-lumen drainage
hose 532b. In the drainage hose, a guidewire 533 is introduced.
Figure 54 is a cross-section of the sponge drainage device of Figure 53.
Sponge body 531 is attached on
drainage hose 532a above perforation openings 534. Drainage hose 532a tapers
toward a small-lumen
drainage hose 532b. Guidewire 533 is introduced into the drainage hose.
Figures 55 a to h show different variants of a distal end of a sponge drainage
device 551, each in a
corresponding cross-section. Sponge body 551 is attached on a drainage hose
552a above perforation
opening 554. Drainage hose 552a ends in a tip 555. In Figure 55a, a string 556
is attached to tip 555. In
Figure 55b, a string or wire loop 557 is attached to tip 555. In Figure 55c, a
string 556 is attached to tip
555. Here, however, the tip has a channel 558, through which a guidewire 553
can be conducted. In
Figure 55d, sponge body 551 is designed as a tip at its distal end. Here, too,
the sponge body has a
channel 558, through which a guidewire was installed. In Figure 55e, sponge
body 551 is also designed
as a tip at the distal end. Sponge body 551 has a channel 558, through which a
guidewire 553 was
installed. To the sponge body, a string or wire loop 57 is attached. In Figure
55f, a grasping bead 559 is
attached to tip 555. In Figure 55g, at the tip, an eyelet 5510 is attached,
through which a string 5511
was pulled. In Figure 55h, grasping bead 559 lies in sponge body 551.
Figures 56 a to fare different representations of a drainage hose 562a and
pointed top-seated
attachments 5612. Figure 56a is a representation of a drainage hose 562a and a
pointed top-seated
attachment 5612. The pointed top-seated attachment has, at its distal end, a
grasping bead 569, at the
proximal end a screw string 5612a. Figure 56b is a representation, in which
pointed top-seated
attachment 5612 is screwed to drainage hose 562a. Figure 56c is a longitudinal
section of Figure 56a
with drainage hose 562a and pointed top-seated attachment 5612. Figure 56d is
a longitudinal section
of Figure 56c with pointed top-seated attachment 5612 screwed onto drainage
hose 562. Figure 56e is a
longitudinal section of pointed top-seated attachment 5612 which is screwed
onto drainage hose 562a
and is equipped with a transverse channel 5612b. Figure 56f is a longitudinal
section of a variant of
34
CA 02829512 2013-09-09
pointed top-seated attachment 5612 which is screwed onto drainage channel
562a. The pointed top-
seated attachment is equipped with a channel 5612c. Through channel 5612c and
the drainage hose, a
guidewire 563 is inserted.
Hereinafter, new insertion instruments are described which are suitable for
vacuum endoscopy.
Endoscopic insertion or grasping instruments are used for the placement of
vacuum drainage devices.
The placement can either be made using an orthograde forward-push technique or
a pull-(through)
technique. When using orthograde placement, an endoscopic insertion instrument
is introduced into
the working channel of the endoscope and an outer working channel. It is
performed on the distal end
of the endoscope. The placement of the drainage device using the pull-
(through) technique is used, if
the wound to be treated can, on the one hand, be endoscopically reached from
the inside via a natural
or artificial access route and, on the other hand, an additional external
access route, for example in the
form of an external fistula, exists.
The pull-(through) technique is also used, if the endoscopic vacuum therapy is
used in combination with
open or laparothoracoscopic surgery (rendezvous procedure). It can also be
used for inserting
conventional drains in laparoscopy.
Based on a therapy example in the case of esophageal leakage with outward
fistulization, the pull-
through technique will be explained. Using a guidewire or an endoscope, the
insertion instrument will
be preplaced from the outside above the fistula opening up to the esophagus.
At the same time, an
endoscope is inserted through the mouth into the esophagus and moved forward
to the leakage point.
When the insertion instrument has arrived at the leak of the esophagus, it is
grasped using a loop and
moved back out retrograde through the mouth. The insertion instrument will be
coupled and attached
by its attachment mechanism to the distal end of the fluid communication
element, the pointed top-
seated attachment or the sponge body. Under endoscopic vision, the insertion
instrument is then
subjected to a pull, the drainage occurs subject to pull by way of the mouth
into the esophagus. The
exact positioning is endoscopically controlled via the esophagus. The
insertion instrument will be
detached from the coupling to the drainage device and removed by further
pulling. If the tip of the fluid
communication element, the pointed top-seated attachment or the sponge body is
reinforced by a
string, the maneuver above can be performed using the string subject to
application of the above
technique.
The application of the pull-(through) procedure is particularly advantageous
if a drainage device design
was selected, in which the sponge body lies in the central section of the
fluid communication element.
In that case, the sponge body can be positioned by pulling on one end of the
fluid communication
element. Aspiration is then possible via only one leg of the fluid
communication element,
simultaneously via both legs or alternating.
The insertion instrument consists of a bead grabber. In a plastic sleeve, a
metal or plastic core is
introduced. The distal end of the core splits into two or a plurality of
leaves. At the distal end of the
core, an outward tension of the leaves exists so that it opens blossom-like
when it emerges from the
distal end of the sleeve and closes during retraction into the sleeve. At
their ends, the leaves are molded
spoon-like, so that upon closing of the core, a spherical or lenticular cavity
forms. At the distal end, after
closing, a small opening remains. Into the blossom-like opened core, the
grasping bead of the pointed
CA 02829512 2013-09-09
top-seated attachment, the fluid communication element or the fluid collection
element can be
introduced. When the core is closed, the bead is firmly seated. During
opening, it detaches easily again
and insertion instrument and grasping bead are uncoupled.
It is particularly advantageous if the insertion instrument is designed to
receive a guidewire. The bead
grabber may be introduced into the working channel of an endoscope. The
insertion instrument is
particularly 80 cm to 250 cm long.
An additional insertion instrument consists of a hook. Into a plastic sleeve,
a wire-like metal or plastic
core is introduced. At the distal end, the core is provided with a hook, by
means of which a string loop or
an eyelet can be grasped. After release of the hook from the sleeve, the
string loop or eyelet of the
pointed top-seated attachment, the fluid communication element or the fluid
collection element can be
attached by retracting the hook. Upon opening of the hook, the connection
releases again. It is
particularly advantageous to introduce a guidewire into the insertion
instrument. The hook can be
inserted into the working channel of an endoscope.
Furthermore, it has been found to be helpful to provide a grasping bead,
string-wire loop, eyelet and/or
a string, attached tension-proof, at the tip, in the latter case, the tip
having a transverse channel, into
which the string can be introduced.
Figures 57 a to f are different representations of an endoscopic insertion
instrument 5713a/b, by means
of which a grasping bead 579 can be grasped. Figure 57a is a representation of
an opened instrument.
Out of a sleeve 5713a, a dual-leaf core 5713b, which has opened, is conducted
out. Moreover, a
guidewire 573 exits from the sleeve. In Figure 57b, the guidewire 573 is
retracted, the grasping bead 579
is grasped using core 5713b. Figure 57c shows how the grasping bead was
grasped. The core 5713b was
retracted into sleeve 5713a; during this process, core 5713b has closed. In
Figure 57d, the closed core
5713b is represented having grasped grasping bead 579 and being retracted into
sleeve 5713a. Figure
57e is a longitudinal section of 57a with sleeve 5713a, opened core 5713b,
guidewire 573 and grasping
bead 579. Figure 57f is a longitudinal section of 57d. The closed core 5713b
with the grasped grasping
bead 579 has been retracted into sleeve 5713a.
Figures 58 a to e are different representations of an additional endoscopic
insertion instrument, by
means of which an eyelet 5810 can be grasped. Figure 58a is a representation
of the opened instrument.
Out of a sleeve 5814a, a hook 5814b is conducted out. Moreover, a guidewire
583 is conducted out of
the sleeve. In Figure 58b, the guidewire 583 is withdrawn, the eyelet 5810 is
grasped using hook 5814b.
Figure 58c shows how the hook was retracted into sleeve 5814a using the
grasped eyelet 5810. Figure
58d is a longitudinal section of the insertion instrument of Figure 58a, with
sleeve 5814a, hook 5814b,
guidewire 583 and eyelet 5810. Figure 58e is a longitudinal section of Figure
58c. The hook 5814b has
been retracted back into the sleeve 5814a using the grasped eyelet 5810.
Figure 59 is a representation of insertion aid 591 with a sleeve 592 for
attachment to a distal end of an
endoscope. Insertion aid 591 is beveled at its distal end, the longer side of
the bevel coming to lie on the
endoscope, in order to avoid injury during insertion of the endoscope. At the
proximal end, a valve 593
is located to prevent leakage of the examination gas.
60 is a representation with 2 insertion aids 601 of different sizes.
36
CA 02829512 2013-09-09
Figure 61 shows a longitudinal section of an insertion aid 611, an attachment
sleeve 612 with valve 613.
Figure 62 shows a representation of an insertion aid 621 having an attachment
sleeve 622 at a distal end
of an endoscope 624
Figure 63 is a representation of an insertion aid 631 having an attachment
sleeve 632 at a distal end of
an endoscope 634. Into the insertion aid, endoscopic forceps 635 were
introduced.
37